Composition for prostaglandin transporter inhibition and related therapeutic applications
a prostaglandin transporter and inhibitor technology, applied in the field of compositions for inhibiting the prostaglandin transporter (pgt), can solve the problems of increasing side effects of synthetic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
f the Composition
[0037]The invention discloses a composition comprising 30-60% w / w Allium sativum extract, 10-30% w / w Beta vulgaris extract, 5-15% w / w Nigella saliva extract and 10-30% w / w Terminalia arjuna extract for the inhibition of PGT. The abovementioned extracts are reported to elicit cardioprotective and anti-hypertensive effects individually. Some of the medicinal properties of the above extracts are mentioned herein below:
[0038]Allium salivum is a well known plant used in the traditional system of Ayurveda. It is reported to elicit many therapeutic effects against different disorders which include cardiovascular diseases, regulating blood pressure, lowering blood sugar and cholesterol levels. It is also effective against bacterial, viral, fungal and parasitic infections and is reported to enhance the immune system. The plant also has anti-tumor properties (Ayaz and Alpsoy, Garlic (Allium sativum) and traditional medicine, Turkiye Parazitol Derg. 2007; 31(2):145-9). It hous...
example 2
n of Prostaglandin Transporter
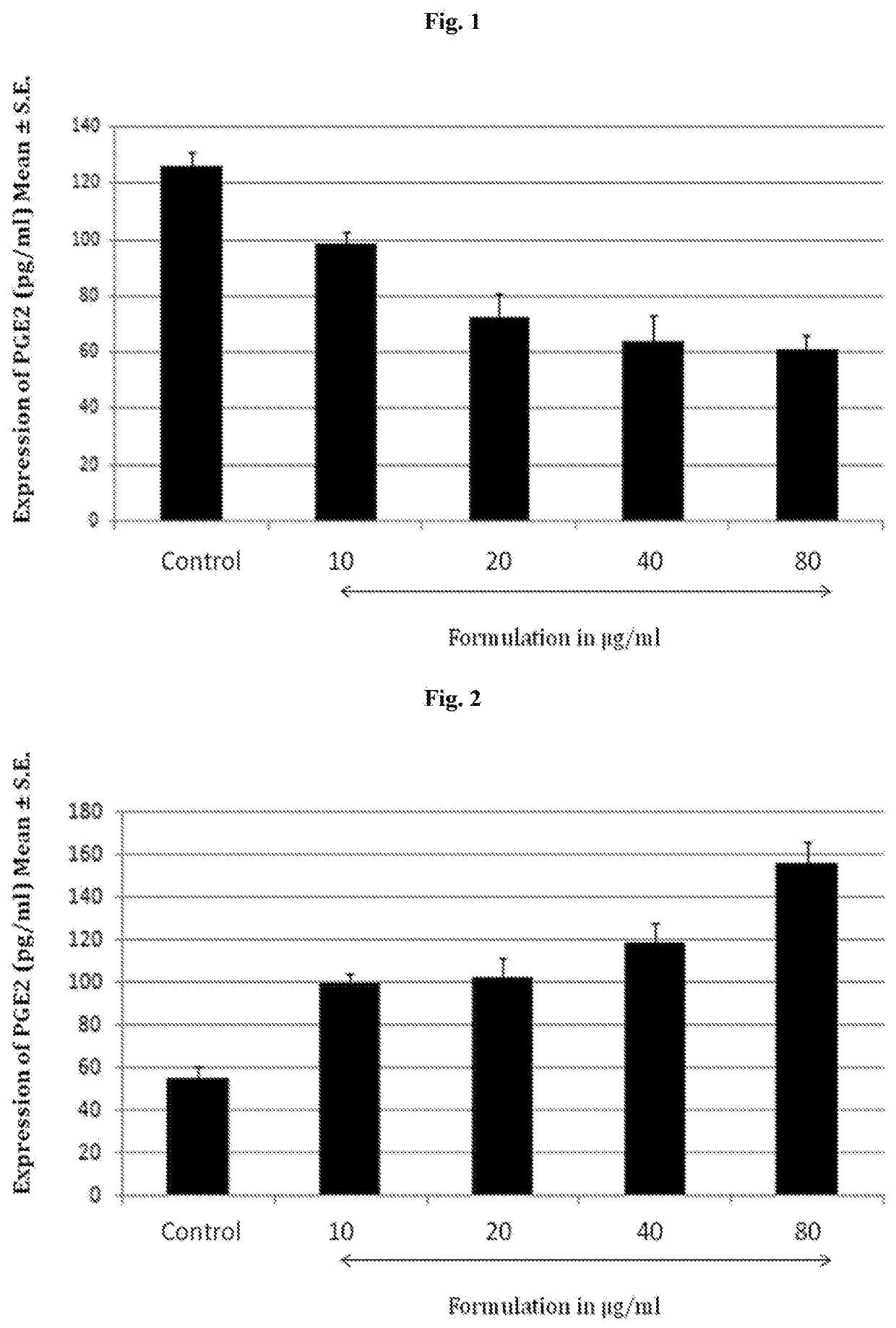
[0043]The formulation was evaluated for its effect inhibiting the prostaglandin transporter (PGT). Kidney cell line, MDCK were seeded onto six-well plates at 30% confluence. Three days later, they were treated with 10 μM bradykinin (to increase endogenous PGE2 synthesis, available in Sigma-Aldrich) in the presence of vehicle (DMSO) at 37° C. for various durations. The formulation was added at graded concentration simultaneously to the cell culture and it was further incubated for 24 hours. Media were collected for measurements of extracellular concentrations of PGE2. Cells were washed with phosphate-buffered saline twice, lysed with 250 μl of phosphate-buffered saline containing 0.1 M HCl and 0.1% Triton X-100 at room temperature for 15 min, and scraped off the plates. Cell suspensions were pipetted up and down for several times to ensure thorough lysing. Cell lysates were collected and centrifuged at 10,000 g 4° C. for 10 min. Supernatants were collect...
example 3
rtensive Effects of the Formulation
[0048]Methods
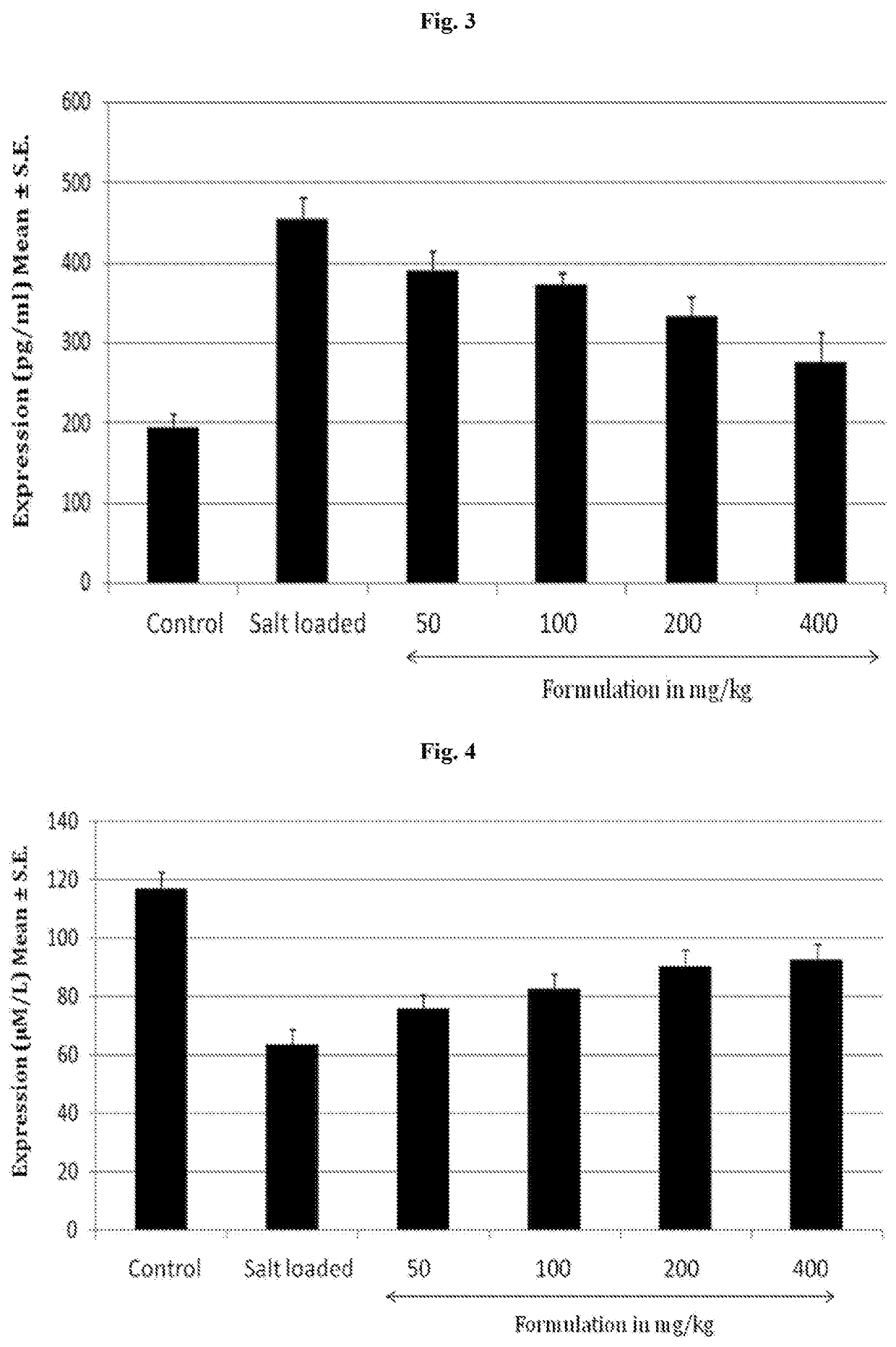
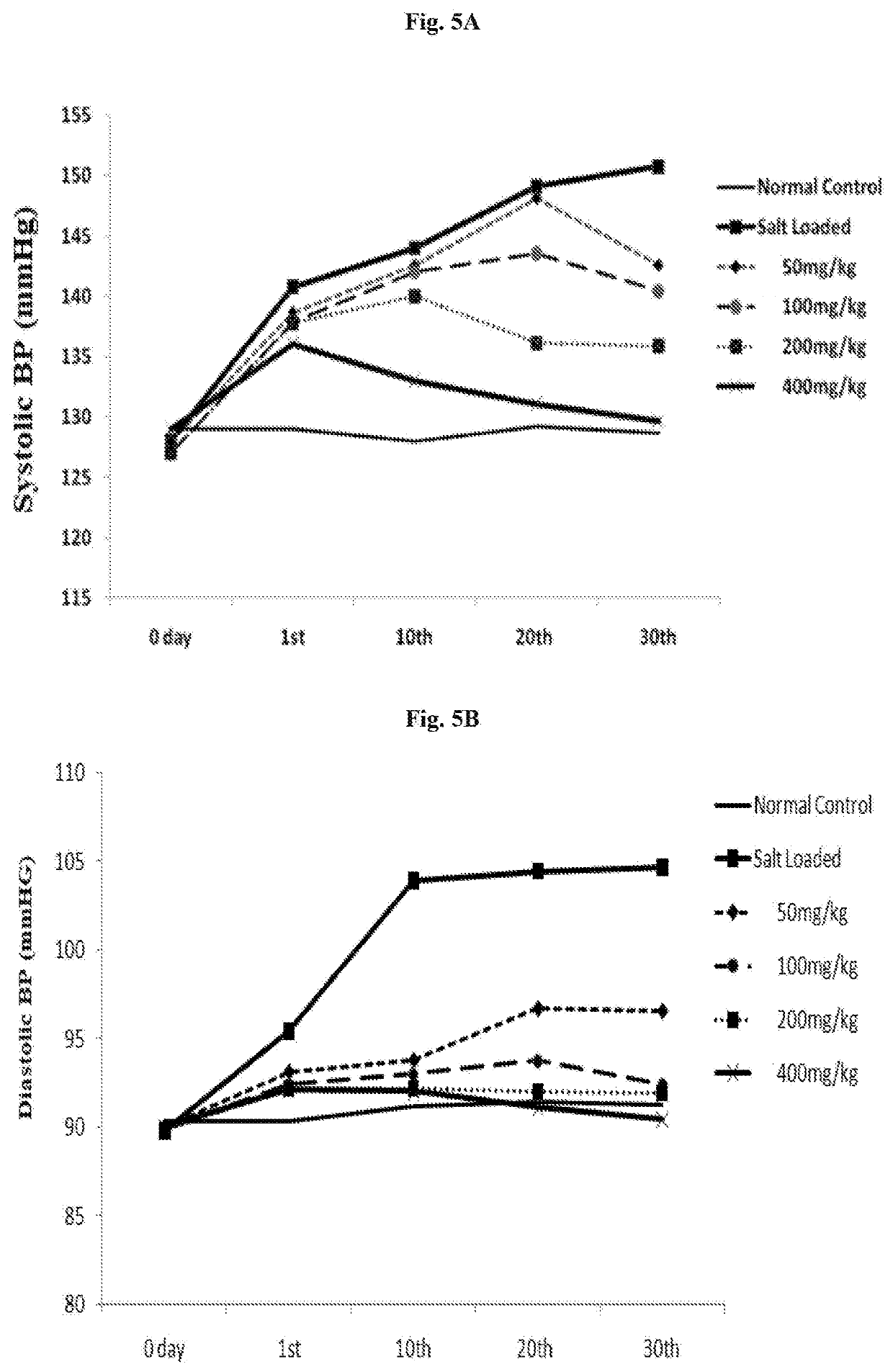
[0049]Normotensive wistar rats were used for the experiments. The rats were spontaneously induced hypertension by the administration of DOCA (deoxycorticosterone acetate) a synthetic mineralocorticoid derivative, 25 mg / kg s.c. twice a week and 1% w / v NaCl in drinking water for a period of 30 days (Model: DOCA salt induced hypertension in rats). The rats were divided into the following groups containing 6 each:
TABLE 2GroupingGroupDescriptionINormal ControlIISalt loadedIIIFormulation at 50 mg / kg bodyweightIVFormulation at 100 mg / kg bodyweightVFormulation at 200 mg / kg bodyweightVIFormulation at 400 mg / kg bodyweight
[0050]The following parameters were estimated to evaluate the anti-hypertensive effects of the formulation[0051]Expression of PGT[0052]Nitric oxide estimation[0053]Blood Pressure[0054]Heart Rate[0055]Mean Arterial Pressure[0056]Urine Volume
[0057]Expression of PGT
[0058]Solute carrier organic anion transporter family, member 2A1 i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| systolic and diastolic pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| diastolic pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com