Methods for diagnosing and treating prostate cancer
a prostate cancer and prostate cancer technology, applied in the direction of lysates, instruments, drug compositions, etc., can solve the problems of poor specificity of psa test, patient discomfor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
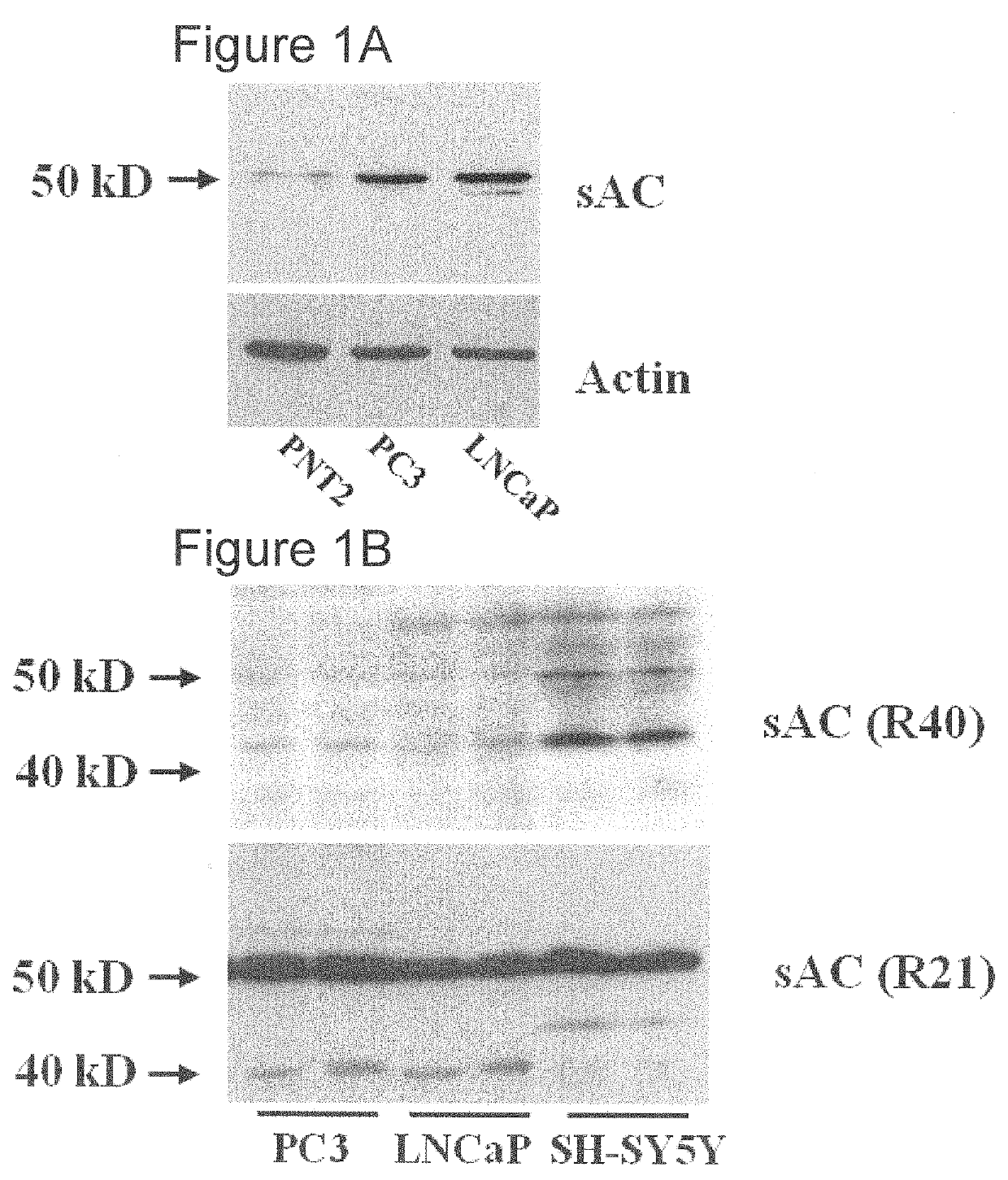
[0044]This example demonstrates that sAC protein is overproduced in prostate cancer cells.
[0045]The sAC gene expression, sAC protein subcellular localization, and sAC protein distribution in both benign and malignant prostate tissue samples were examined. Immunohistochemical staining using a mouse monoclonal sAC antibody (R21) was performed on both tumor and benign tissue from 12 radical prostatectomy specimens. Two tumors were well-differentiated (Gleason score 6), 7 were moderately differentiated (Gleason score 7), and 3 were poorly differentiated (Gleason score 8-10).
[0046]Briefly, five micron-thick sections of the formalin-fixed paraffin-embedded tissue were deparaffinized and stained using a Bond III Autostainer (Leica Microsystems, Buffalo Grove, Ill.) and the manufacturer's Heat-Induced Epitope Retrieval 1 protocol with supplied reagents. Mouse monoclonal R21 sAC antibody (CEPBiotech, Inc, R21-IHC, Tamarac, Fla.) was used at a dilution of 1:750 as previously described (Zippin...
example 2
[0052]This example demonstrates that sAC protein is overproduced in prostate cancer cells.
[0053]Prostate cancer cases were retrospectively identified from the database of the Division of Surgical Pathology, Weill Cornell Medical College. Tissue microarrays (TMAs) were constructed from the archival formalin-fixed, paraffin-embedded tissue samples using 0.6 mm cores, with each area represented in triplicate. When possible, areas of benign prostatic tissue, high grade prostatic intraepithelial neoplasia (HGPIN), and invasive prostatic adenocarcinoma were all sampled from each case; however, in some cases not all tissue types were present for sampling / evaluation.
[0054]Immunohistochemical staining for three sAC antibodies (R21, R40, and R52) was performed on the TMA slides. The TMAs included 50 samples of benign prostatic tissue, 35 samples of HGPIN, 65 samples of localized prostatic adenocarcinoma, and 25 samples of neuroendocrine prostate cancer (NEPC), castration resistant prostate ca...
example 3
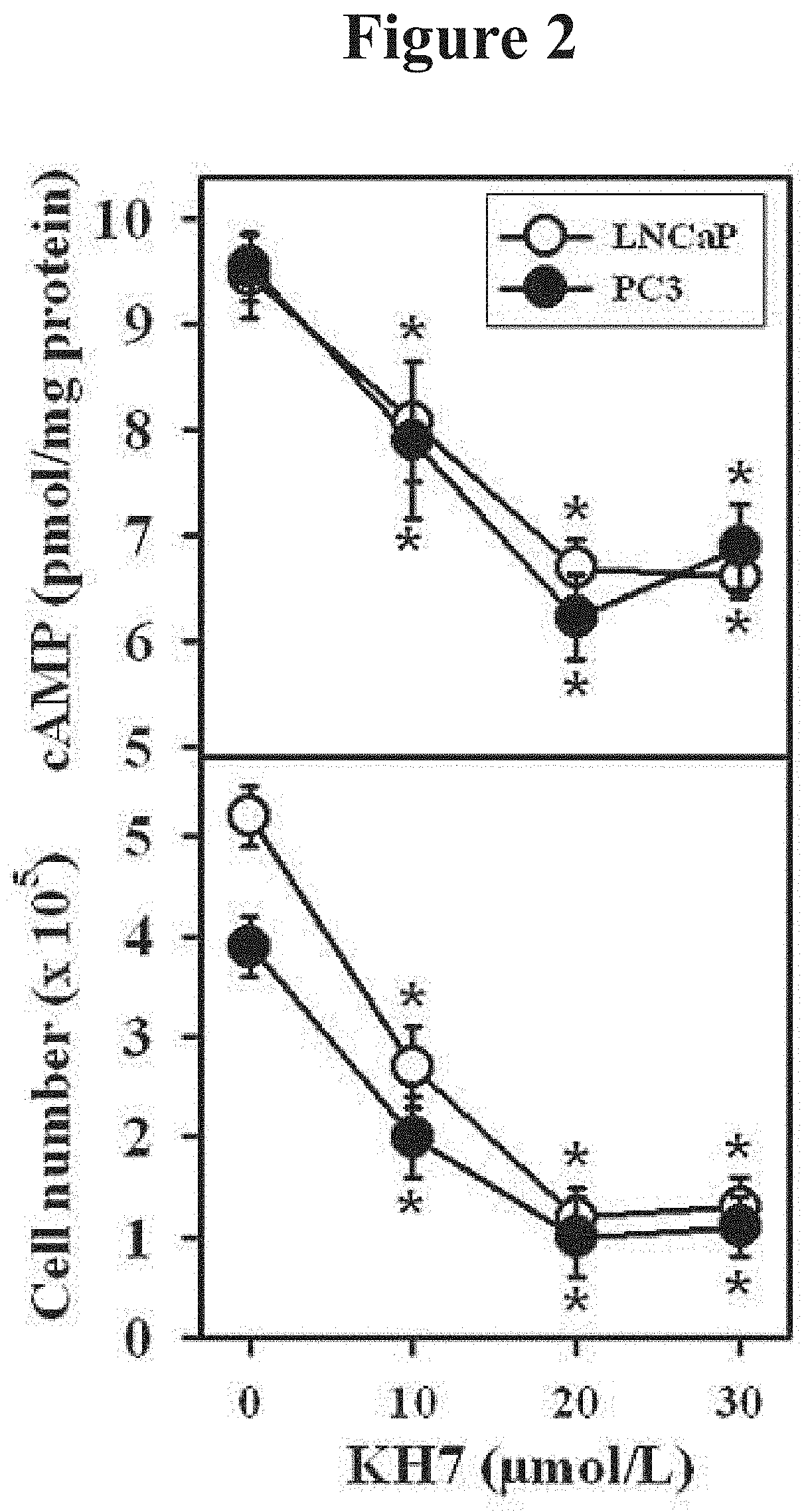
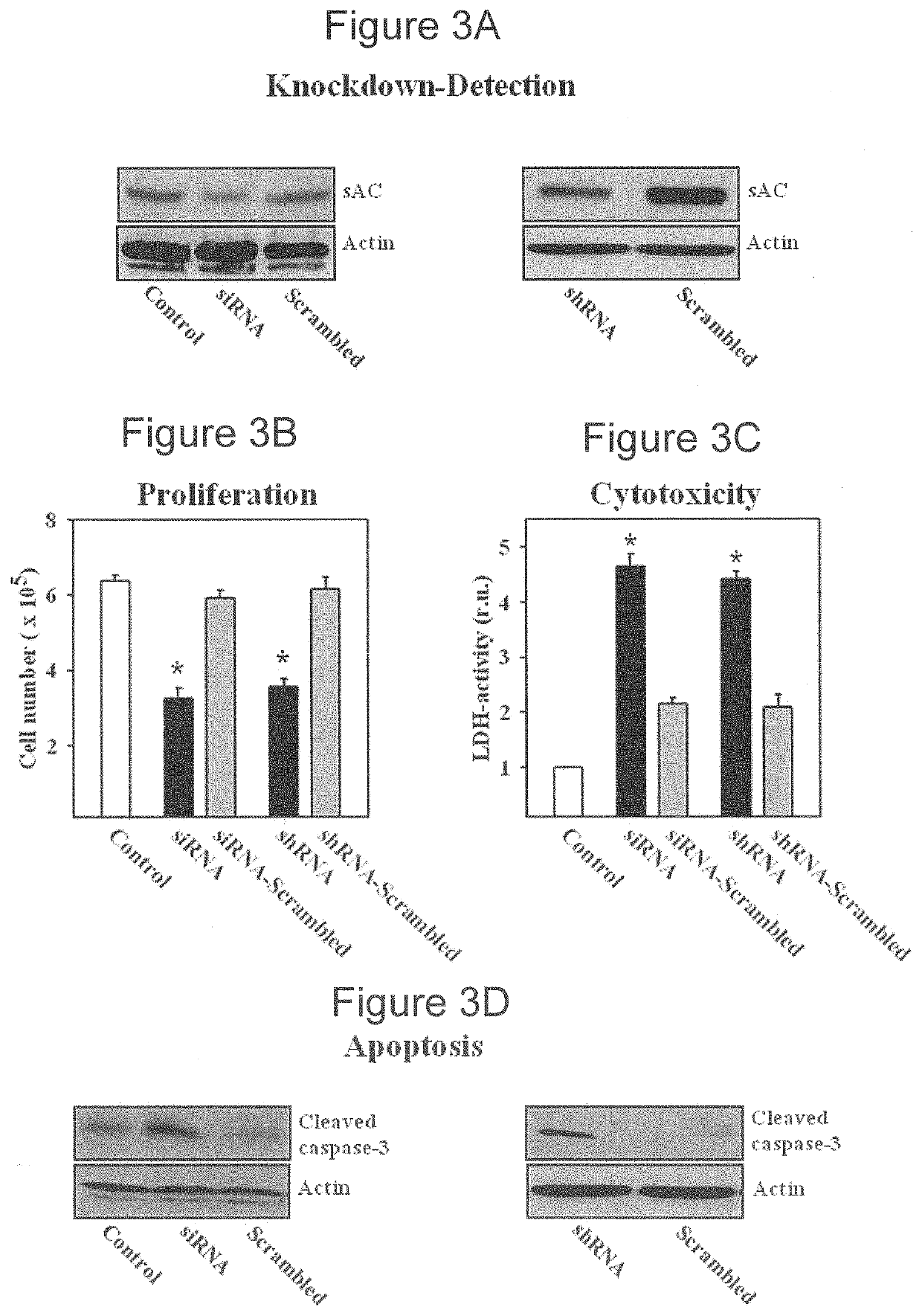
[0061]This example demonstrates a method of inhibiting proliferation of prostate cancer cells by suppressing the activity of soluble adenylyl cyclase (sAC) protein.
[0062]The androgen-sensitive LNCaP (ATCC-Nr. CRL-1740D) human prostate carcinoma cell line, the androgen-insensitive PC3 (ATCC-Nr. CRL-1435D) human prostate carcinoma cell line, and neuroblastoma cell line SH-SY5Y (ATCC-Nr. CRL-2266) were purchased from the American Type Culture Collection, and human normal prostate epithelial cell line PNT2 was purchased from Sigma-Aldrich (Cat. Nr. 95012613). Cells were expanded and frozen in aliquots within four weeks of purchase. Cells were thawed and cultured for no more than three further passages. PNT2 cells were cultured in medium RPMI1640 supplemented with 10% fetal calf serum, glutamine and antibiotics. All other cells were cultured in Dulbecco's modified Eagle's medium that was supplemented with 5% fetal calf serum, glutamine, and antibiotics. The cells (1.5×105) were seeded in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com