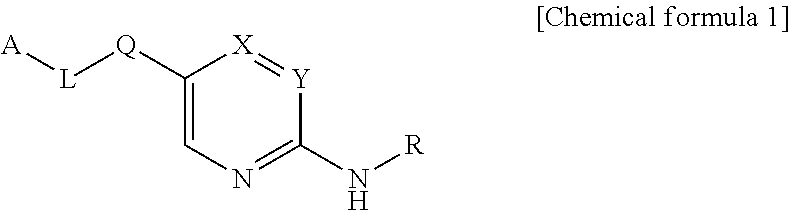
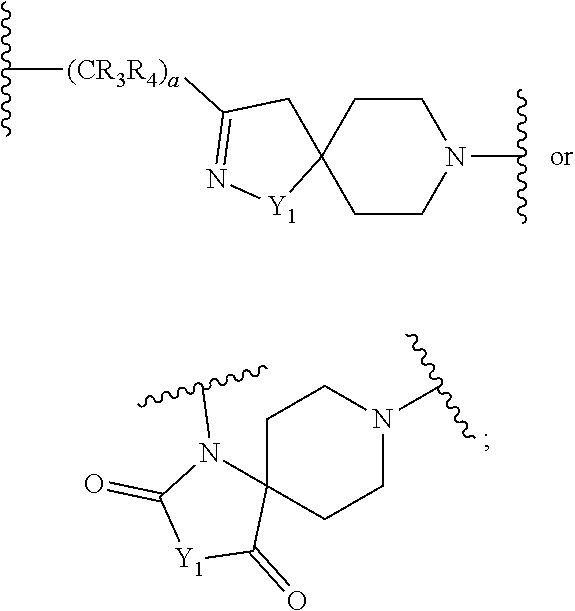
Novel compounds as autotaxin inhibitors and pharmaceutical compositions comprising the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1-1
[Preparation Example 1-1] Preparation of 5-bromo-N-(2,3-dihydro-1H-inden-2-yl)pyrimidin-2-amine (Compound im-1a)
[0225]
[0226]5-Bromo-2-chloropyrimidine (2.0 g, 10.3 mmol), 2-aminoindane (1.6 mL, 12.4 mmol), and N,N-diisopropylethylamine (4.5 mL, 25.8 mmol) were dissolved in ethanol (10 mL) and stirred at 90° C. for 2 hours. Upon the completion of the reaction, the mixture was cooled to room temperature, and the resulting solid was filtered, washed with ethanol (20 mL), and dried to obtain the title compound im-1a as a beige solid (2.2 g, 72%).
[0227]MS m / z: 291 [M+1]+
[0228]1H NMR (DMSO-d6, 400 MHz), δ ppm: 8.40 (s, 2H), 7.81 (d, 1H), 7.22-7.13 (m, 4H), 4.56-4.51 (m, 1H), 3.23 (dd, 2H), 2.88 (dd, 2H)
preparation example 1-2
[Preparation Example 1-2] Preparation of 5-bromo-N-{[3-(trifluoromethoxy)phenyl]methyl}pyrimidin-2-amine (Compound im-1b)
[0229]
[0230]Except that 3-trifluoromethoxy benzylamine is used instead of 2-aminoindane, the reaction was carried out in the same manner as Preparation example 1-1 to obtain the title compound im-1b as a light brown liquid.
[0231]MS m / z: 349 [M+1]+
[0232]1H NMR (DMSO-d6, 400 MHz), δ ppm: 8.39 (s, 2H), 8.11 (t, 1H), 7.44 (t, 1H), 7.33 (d, 1H), 7.25 (s, 1H), 7.22 (d, 1H), 4.52 (d, 2H)
preparation example 1-3
[Preparation Example 1-3] Preparation of 5-bromo-N-[4-(3-chlorophenyl)cyclohex-3-en-1-yl]pyrimidine-2-amine (Compound im-1c)
[0233]
(Step 1) Preparation of tert-butyl N-(4-hydroxycyclohexyl)carbamate (Compound im-1c-a)
[0234]After dissolving (1R,4R)-4-aminocyclohexan-1-ol (15.0 g, 0.13 mol) in methylene chloride (250 mL), di-tert-butyl dicarbonate (25.6 g, 0.12 mol) diluted in methylene chloride (100 mL) and triethylamine (45.4 mL, 0.33 mol) were slowly added dropwise thereto in order under stirring at 0° C., followed by stirring for 14 hours at room temperature. The reaction mixture was diluted with distilled water (300 mL) and stirred for 10 minutes to terminate the reaction, followed by extraction with methylene chloride. The organic layer was washed with distilled water and saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain the title compound im-1c-a as a pink solid (24.5 g, 97%).
[0235]MS m / z: 216 [M+1]+
[0236]1H NMR (CDCl3, 400 MHz), δ ppm: 4.35 (br, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com