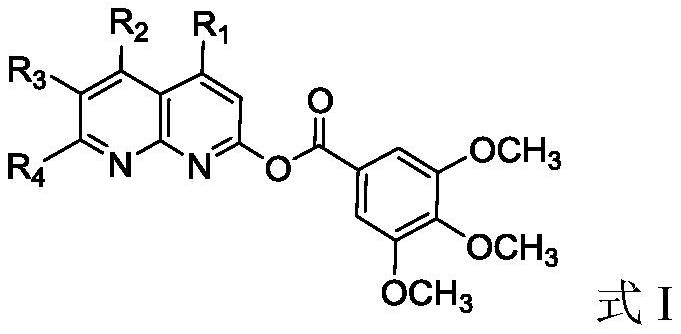
Naphthyridine derivative and application thereof as monoamine oxidase inhibitor
A use, technology of naphthyridine, applied in the field of naphthyridine derivatives and monoamine oxidase inhibitors, to achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
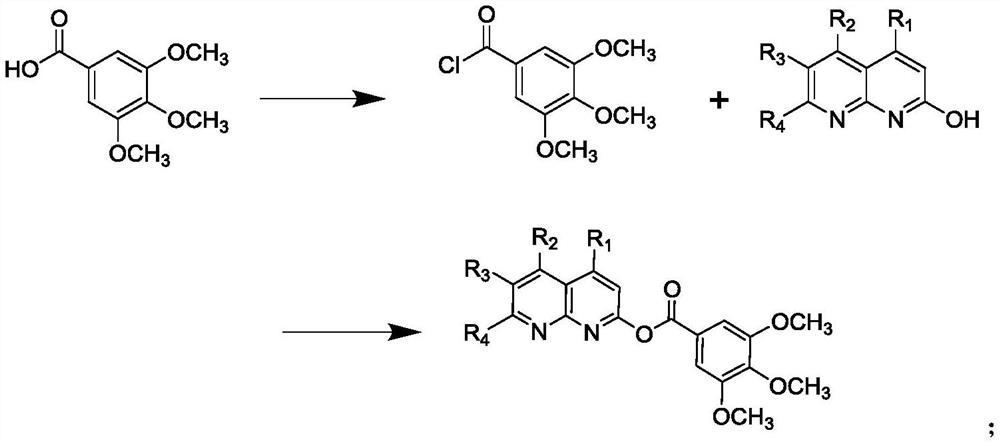
[0030] The preparation of embodiment 1 compound I-1
[0031]
[0032] Step 1): Under nitrogen atmosphere, add 3,4,5-trimethoxybenzoic acid (4.24g, 0.02mol) and 40ml benzene into the reaction kettle, stir to dissolve, and then add SOCl 2 (7.15g, 0.06mol), react at 70-80°C for 6 hours, after the reaction, recover benzene by distillation under reduced pressure, and remove excess SOCl at the same time 2 , directly used in the next reaction without purification;
[0033] Step 2): Under nitrogen atmosphere, add 2-hydroxy-1,5-naphthyridine (3.21g, 0.22mol) and 50ml tetrahydrofuran into the reaction kettle, stir and dissolve, then slowly add the 3,4, 5-Trimethoxybenzoyl chloride tetrahydrofuran solution (20ml) was added dropwise in half an hour, and reacted overnight at room temperature. After the reaction was detected by TLC, tetrahydrofuran was recovered by distillation, and the residue was washed with dichloromethane solution and 10% aqueous sodium carbonate solution, then was...
Embodiment 2-3
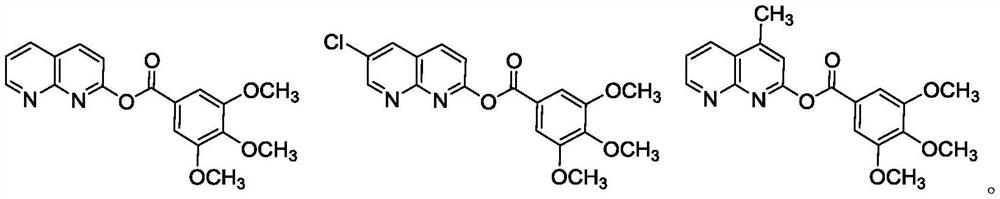
[0035] The preparation of embodiment 2-3 compound I-2 and I-3
[0036] Referring to the method of Example 1, compounds I-2 and I-3 were prepared, and their structure confirmation data are as follows:
[0037]
[0038]
Embodiment 4
[0039] The inhibitory activity of embodiment 4 compound I to monoamine oxidase A and B
[0040]Use 100mM pH 7.4 potassium phosphate buffer to make recombinant human MAO-A into 12.5 μg / mL sample solution, and MAO-B into 75 μg / mL sample solution. Add 20 μL of the test compound solution and 80 μL of monoamine oxidase to a black 96-well plate, mix well, incubate at 37°C in a dark place for 15 minutes, add 200 μM Amplex Red reagent, 2U / mL horseradish peroxidase, 2mM p-hydroxyphenylethylamine (inhibit MAO-A) or 2mM benzylamine (inhibit MAO-B) to initiate the reaction, incubate at 37°C for 20min, measure the fluorescence emission intensity at 590nm on a multi-functional microplate reader with a fixed excitation wavelength of 545nm, buffer with potassium phosphate solution instead of MAO-A or MAO-B as blank; the formula for the inhibitory rate of monoamine oxidase inhibition by the compound is: 100-(IFi) / (IFc)*100, where IFi and IFc are the presence of inhibitors and no inhibitors res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com