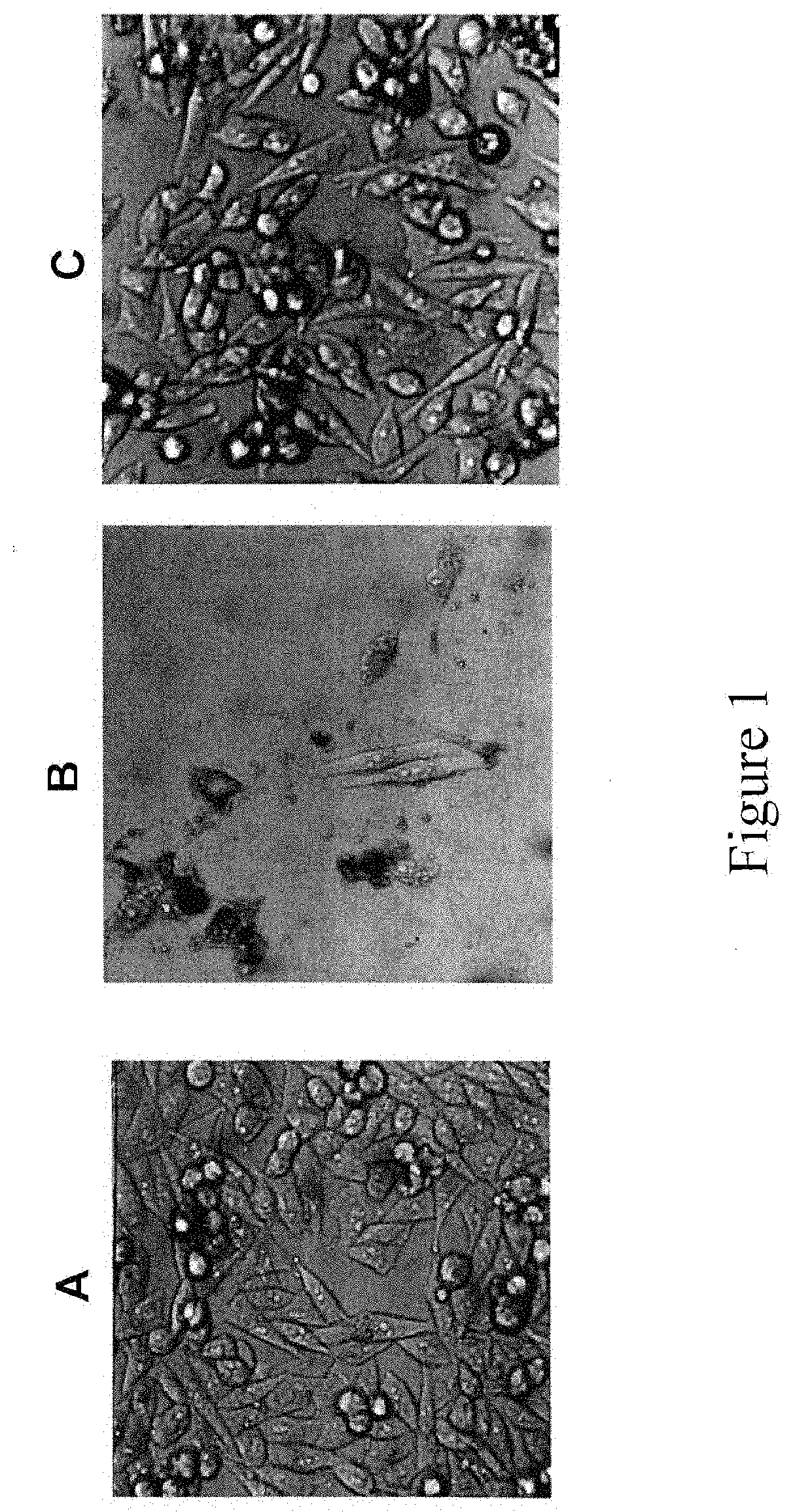
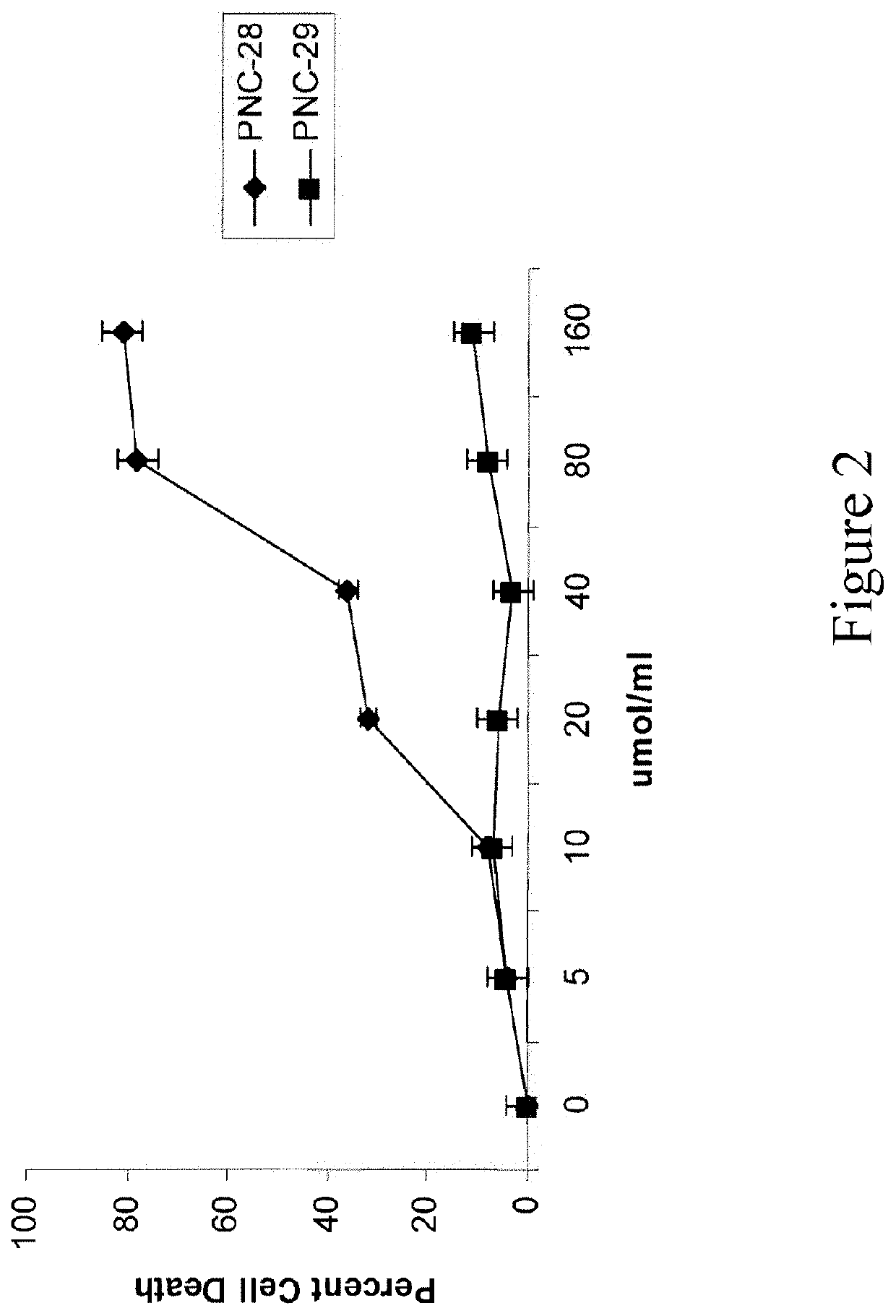
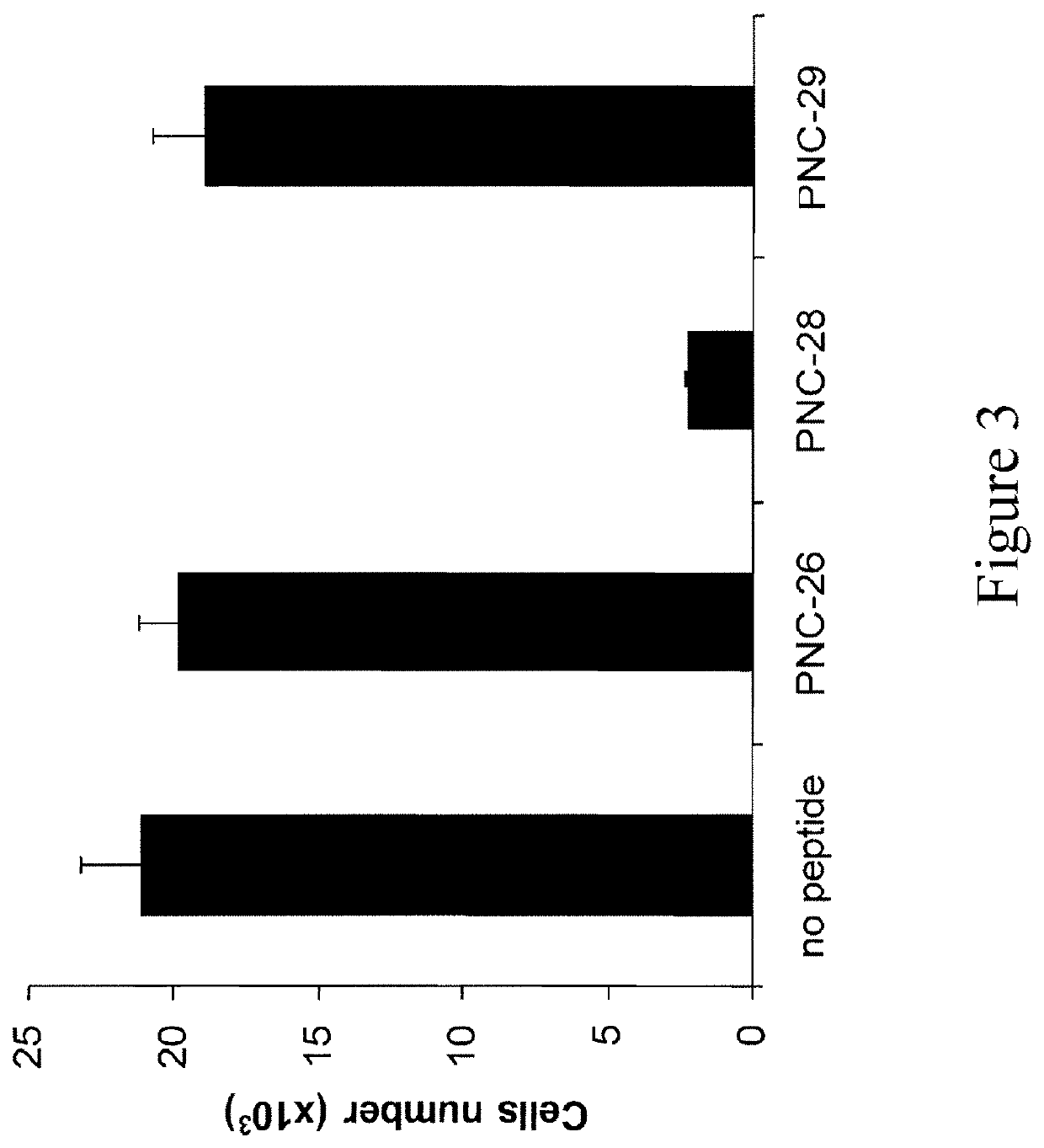
Hdm-2 targeting compositions cause tumor cell necrosis rather than apoptosis of cancer cells
a technology of hdm2 and composition, which is applied in the direction of peptide sources, p53 proteins, instruments, etc., can solve the problems of limited p53-targeting cancer treatments and ineffective p53-targeting cancer treatments at treating various types of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials and Methods
[0143]Peptides.
[0144]The following peptides were synthesized by solid phase methods and were purified to >95% purity (Biopeptides Corp, La Jolla, Calif.): PNC-27 containing residues 12-26 (PPLSQETFSDLWKLL) (SEQ ID NO: 8) from the hdm-2 binding domain of p53 and PNC-28 (ETFSDLWKLL) (SEQ ID NO: 32) containing residues 17-26 from the hdm-2 binding domain of p53, both attached on their carboxyl terminal ends to the transmembrane-penetrating sequence which is related to the reverseomer sequence of the antennapedia sequence, KKWKMRRNQFWVKVQRG (SEQ ID NO: 1), also called MRP; the control peptide PNC-26, containing only residues 12-26 of p53 and no MRP; the control peptide PNC-29, an unrelated peptide from cytochrome P450 (also called X13) (bold) attached to MRP (italics), whose sequence is MPFSTGKRIMLGEKKWKMRRNQFWVKVQRG (SEQ ID NO: 4); and PNC-7, a peptide from the ras-p21 protein containing ras-p21 residues 35-47 (TIEDSYRKQVVID) (SEQ ID NO: 7) attached to the MRP havi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| OD | aaaaa | aaaaa |
| OD | aaaaa | aaaaa |
| OD | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com