Composite air electrode and associated manufacturing method
a technology of air electrodes and manufacturing methods, which is applied in the direction of fuel and primary cells, non-aqueous electrolyte cells, cell components, etc., can solve the problems of reducing the performance of metals, consuming water, and degrading the performance of air electrodes, so as to facilitate the formation of hole-free layers, and reduce the thickness of polymer membranes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
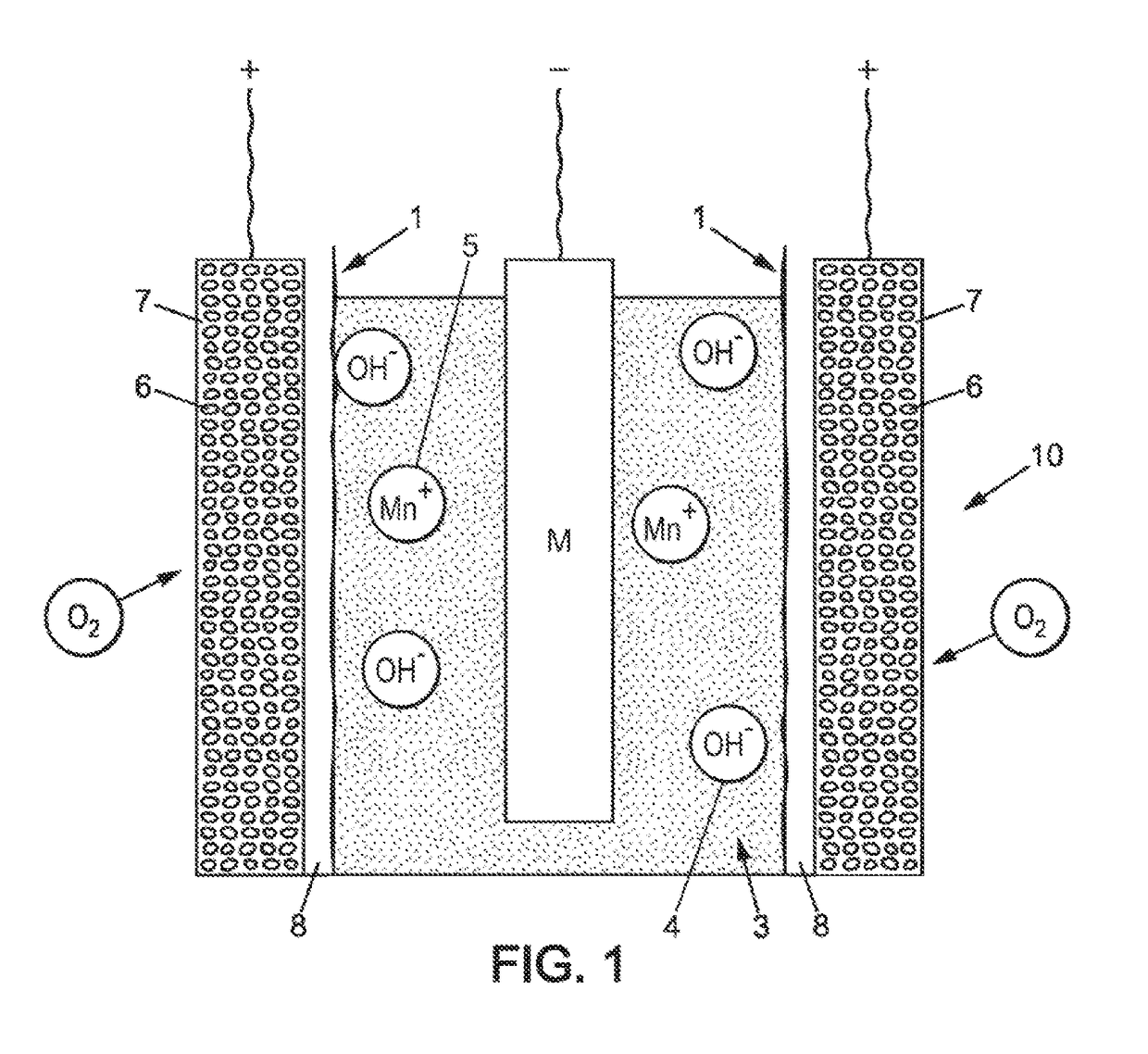
[0064]The invention relates to a method for protecting an air electrode from the negative effects of a liquid electrolyte of basic pH FIG. 1 shows a metal-air electrochemical cell 10 comprising a composite electrode which is an object of the present invention, obtainable by the method presented below. The cell represented in FIG. 1 may be an integral part of a metal-air battery comprising a plurality of electrochemical cells. It is also possible for a battery to comprise only one cell.
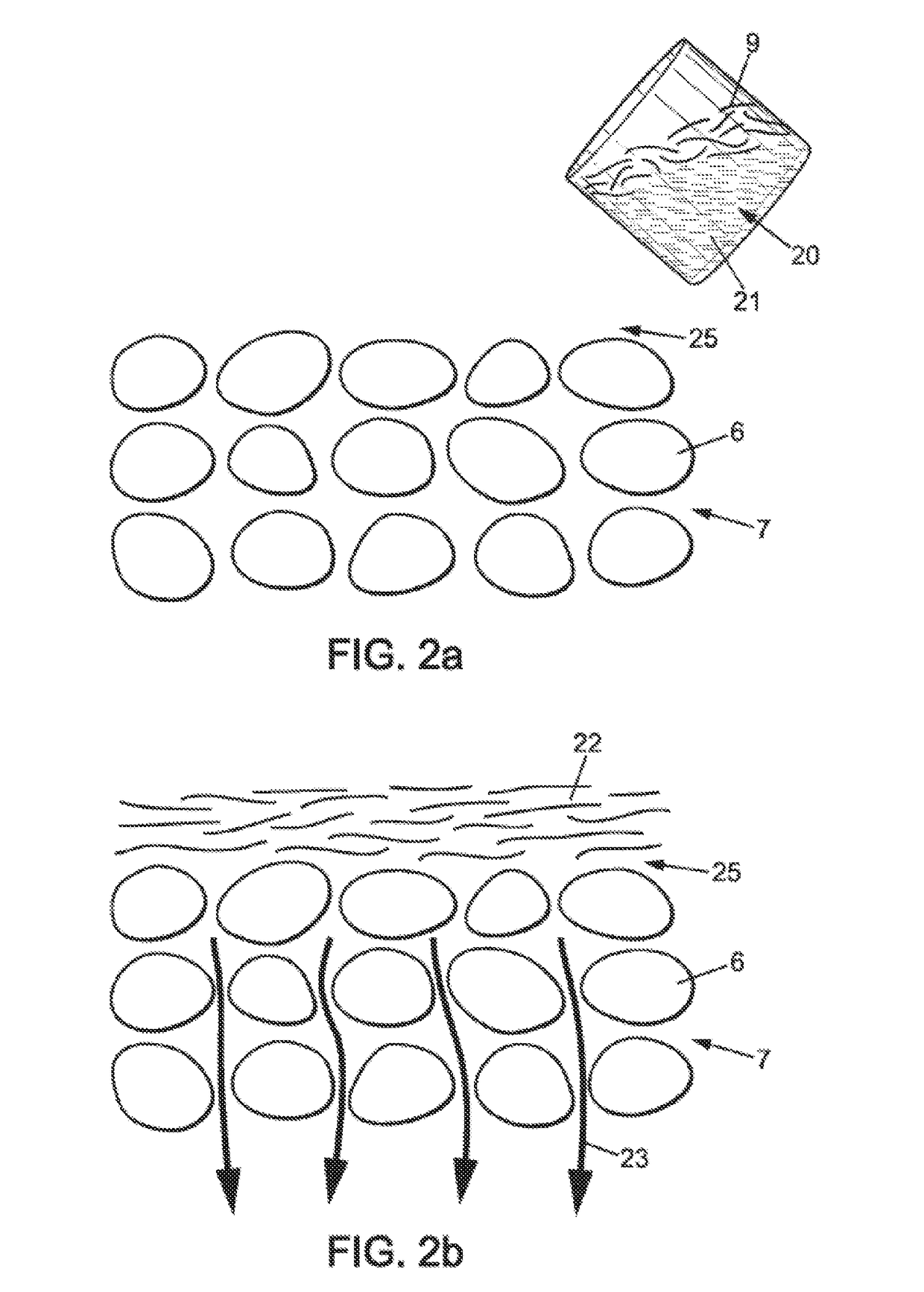
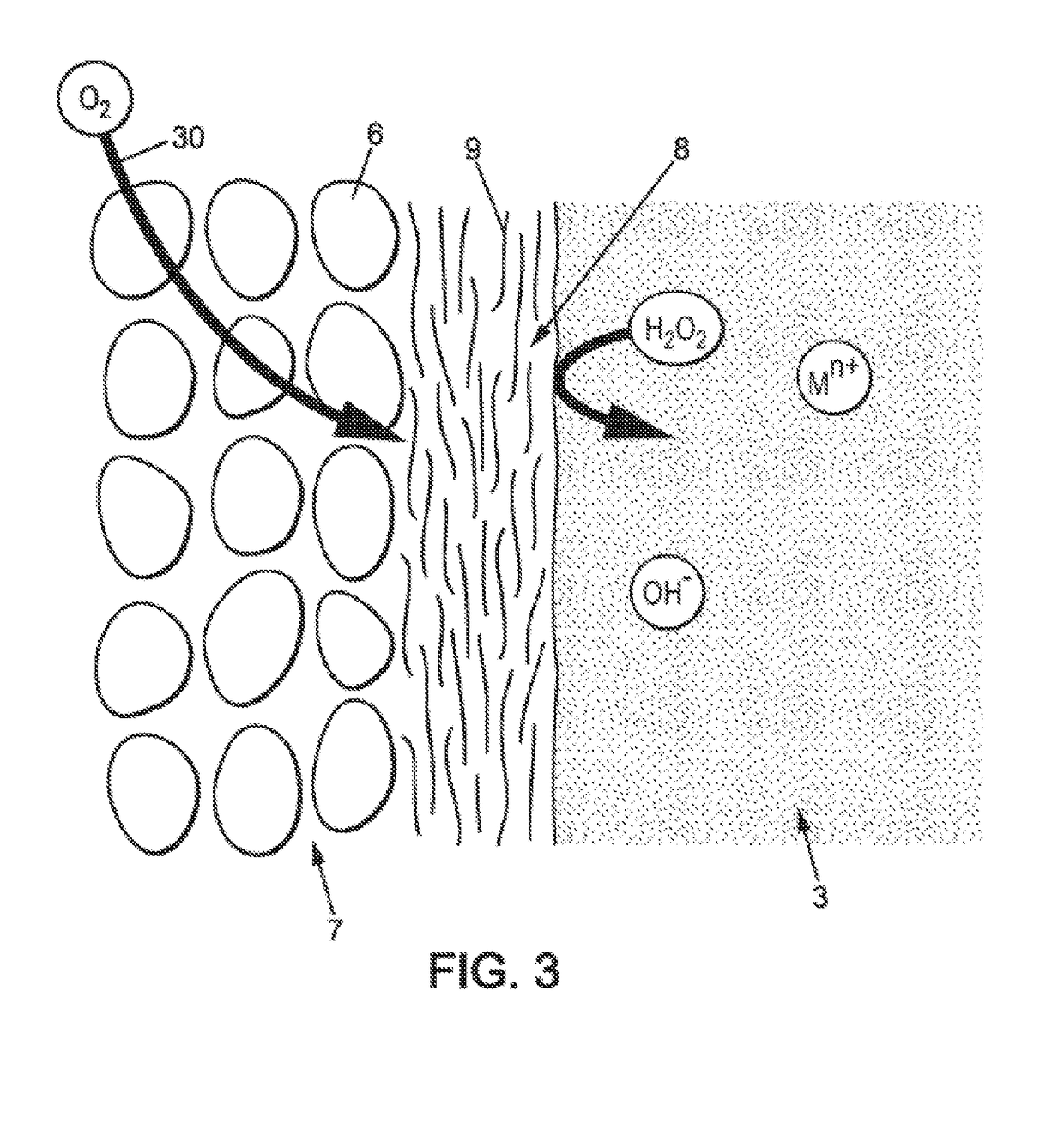
[0065]As shown in FIG. 1, the electrochemical cell 10 comprises two composite electrodes 1, corresponding to air electrodes having a porous structure 7. The porous structure 7 of an air electrode of an electrochemical cell can be obtained from carbon grains 6 joined by a binder. The space between the carbon grains 6 allows air and in particular oxygen contained in the air to flow through the porous structure 7 to a triple interface of air / electrode / OH− ions. This triple interface is the site of oxidati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com