Indium electroplating compositions and methods for electroplating indium
a technology of indium electroplating and composition, applied in the direction of basic electric elements, semiconductor devices, etc., can solve the problems of increasing surface roughness, difficult reproducibility of plate void-free uniform indium of target thickness and smooth surface morphology on metal layers, corrosion of indium in galvanic interaction, etc., to reduce damage on assembled chips
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0054]The method described in Example 1 above was repeated except that the indium electroplating composition included the following components:
TABLE 2COMPONENTAMOUNTIndium sulfate45g / LCitric acid96g / LSodium citrate dihydrate59g / LGuanylthiourea0.75g / L
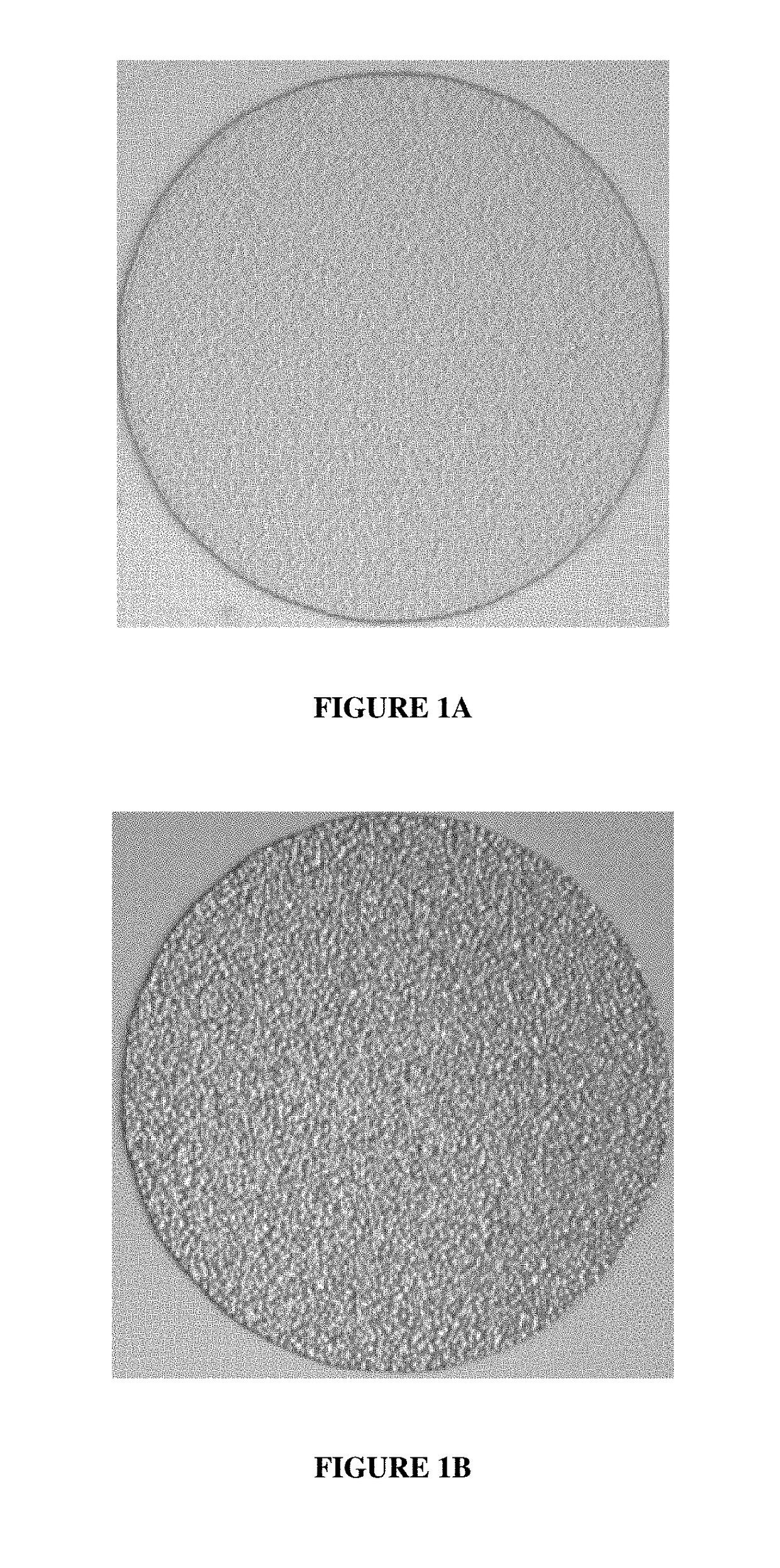
[0055]The nickel plated silicon wafers were immersed in the indium electroplating composition and indium metal was electroplated on the nickel. Indium electroplating was done at 25° C. at a current density of 4ASD for 30 seconds. The pH of the composition was 2.4. The anode was an indium soluble electrode. After indium was electroplated on the nickel layers, the photoresist was stripped from the wafers and the indium morphology was observed. All of the indium deposits appeared uniform and smooth.
[0056]FIG. 2 is an optical microscope image of one of the indium metal deposits electroplated on the nickel layer. The indium deposit appeared smooth in contrast to the indium deposit of FIG. 1B.
example 3
[0057]The method described in Example 1 above was repeated except that the indium electroplating composition included the following components:
TABLE 3COMPONENTAMOUNTIndium sulfate45g / LCitric acid96g / LSodium citrate dihydrate59g / LTetramethyl-2-thiourea0.5g / L
[0058]The nickel plated silicon wafers were immersed in the indium electroplating composition and indium metal was electroplated on the nickel. Indium electroplating was done at 25° C. at a current density of 4ASD for 30 seconds. The pH of the composition was 2.4. After indium was electroplated on the nickel, the photoresist was stripped from the wafers and the indium morphology was observed. All of the indium deposits appeared uniform and smooth.
[0059]FIG. 3 is an optical microscope image of one of the indium metal deposits electroplated on the nickel. The indium deposit appeared smooth in contrast to the indium deposit of FIG. 1B.
example 4
[0060]The method described in Example 1 above was repeated except that the silicon wafers were patterned with photoresist to have rectangular vias having lengths of 50 μm and the indium electroplating composition included the following components:
TABLE 4COMPONENTAMOUNTIndium sulfate45g / LCitric acid96g / LSodium citrate dihydrate59g / L1-allyl-2-thiourea11g / L1synonym = N-allyl-thiourea
[0061]The nickel plated silicon wafers were immersed in the indium electroplating composition and indium metal was electroplated on the nickel. Indium electroplating was done at 25° C. at a current density of 4ASD for 11 seconds. The pH of the composition was 2.4. After indium was electroplated on the nickel, the photoresist was stripped from the wafers and the indium morphology was observed. All of the indium deposits appeared uniform and smooth.
[0062]FIG. 4 is an optical microscope image of one of the indium metal deposits electroplated on the nickel layer. The indium deposit appeared smooth in contrast t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com