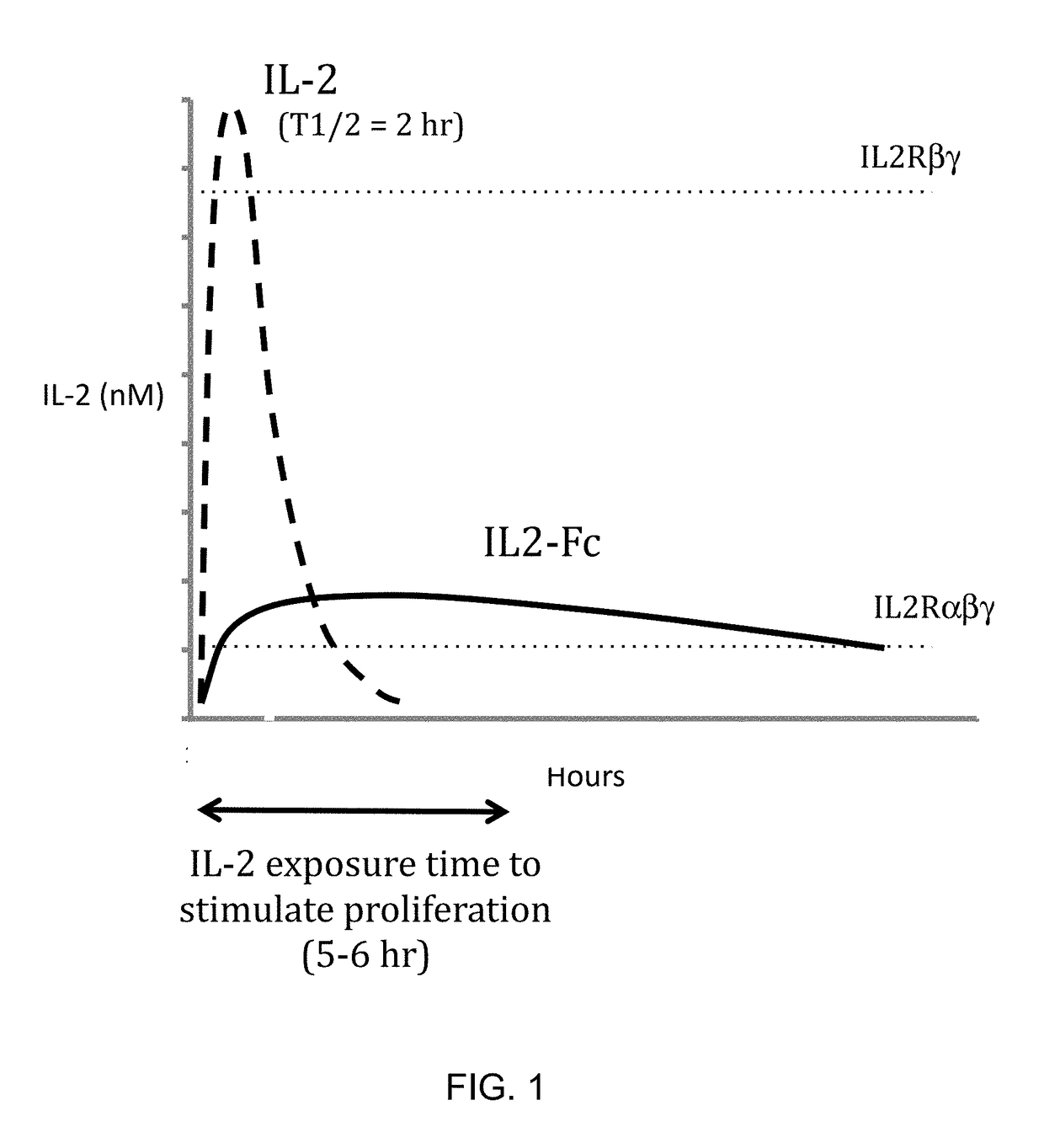
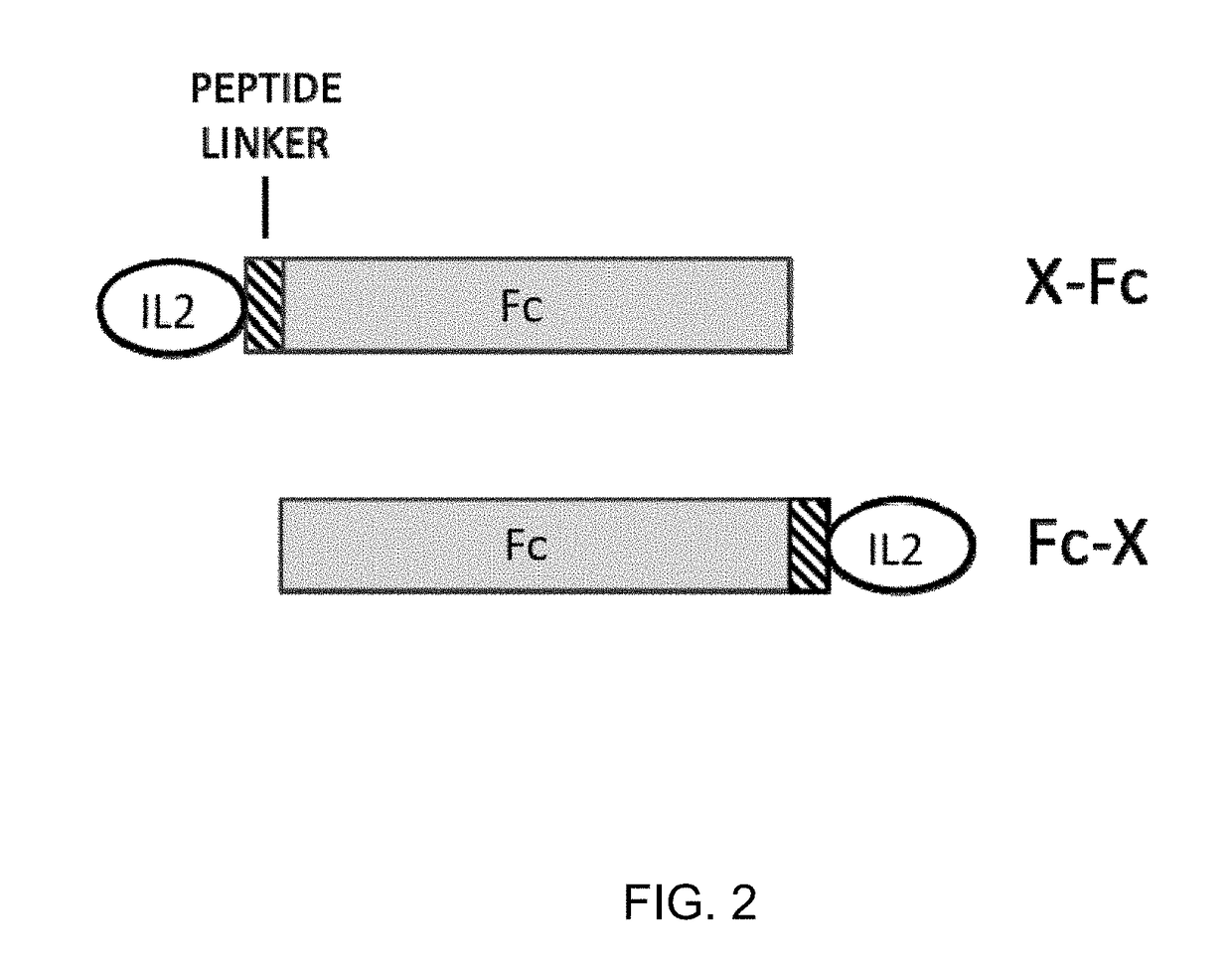
Molecules that selectively activate regulatory t cells for the treatment of autoimmune diseases
a technology of regulatory t cells and molecules, applied in the field of molecules that selectively activate regulatory t cells for the treatment of autoimmune diseases, can solve the problems of autoimmune diseases, invasiveness, and inability to meet the needs of patients' own t cells, and achieve the effects of prolonging the circulating half-life, and increasing the molecular size of fusion proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression, and Purification of IL-2 Selective Agonist-IgG Fc Fusion Proteins
[0091]A cDNA encoding N88RL9AG1 (SEQ ID NO 4) was constructed by DNA synthesis and PCR assembly. The N88RL9AG1 construct was composed of the mouse IgG1 signal sequence, the mature human IL-2 (SEQ ID NO 1) sequence with the substitutions N88R and C125S, a 9 amino acid linker peptide sequence (SEQ ID NO 15), and the Fc region of human IgG1 containing the substitution N297A (SEQ ID NO 2). N88R / IL2 is an IL2 selective agonist with reduced binding to IL2RB and selective agonist activity on IL2Rαβγ receptor-expressing cells (Shanafelt, A. B., et al., 2000, Nat Biotechnol. 18:1197-202). Elimination of the N-linked glycosylation site at N297 on IgG1 Fc reduces Fc effector functions (Tao, M. H., et al., 1989, J Immunol. 143:2595-2601). D20HL0G2 was composed of the mouse IgG1 signal sequence, IL-2 (SEQ ID NO 1) with the substitutions D20H and C125S, and an Fc protein moiety derived from human IgG2 (SEQ ID NO 3). The ...
example 2
tion of Receptor-Binding Activity of N88RL9AG1 and D20HL0G2
[0094]To determine if N88RL9AG1 and D20HL0G2 were properly folded, their affinity to the IL-2 receptor subunits IL2RA and IL2RB was determined by surface plasmon resonance (SPR) using a Biacore T-200 instrument (GE Healthcare). IL2RA and IL2RB extracellular domain proteins and IL-2 protein (R&D Systems, Minneapolis, Minn.) were immobilized on CM-5 Biacore chips by NHS / EDC coupling to final RU (resonance units) values of 30 and 484, respectively. The kinetics of binding to IL2RA was measured at five concentrations of IL2 and N88RL9AG1 ranging from 0.6 nM to 45 nM at a flow rate of 50 ul / minute. The kinetics of binding to IL2RB was measured at five concentrations ranging from 16.7 nM to 450 nM for IL2 and from 14 nM to 372 nM for the Fc fusion proteins at a flow rate of 10 ul / minute. The dissociation constants (Kd) were calculated from the kinetic constants using the Biacore evaluation software version 2.0, assuming 1:1 fit fo...
example 3
ty of N88RL9AG1 and D20HL0G2 on T Cells
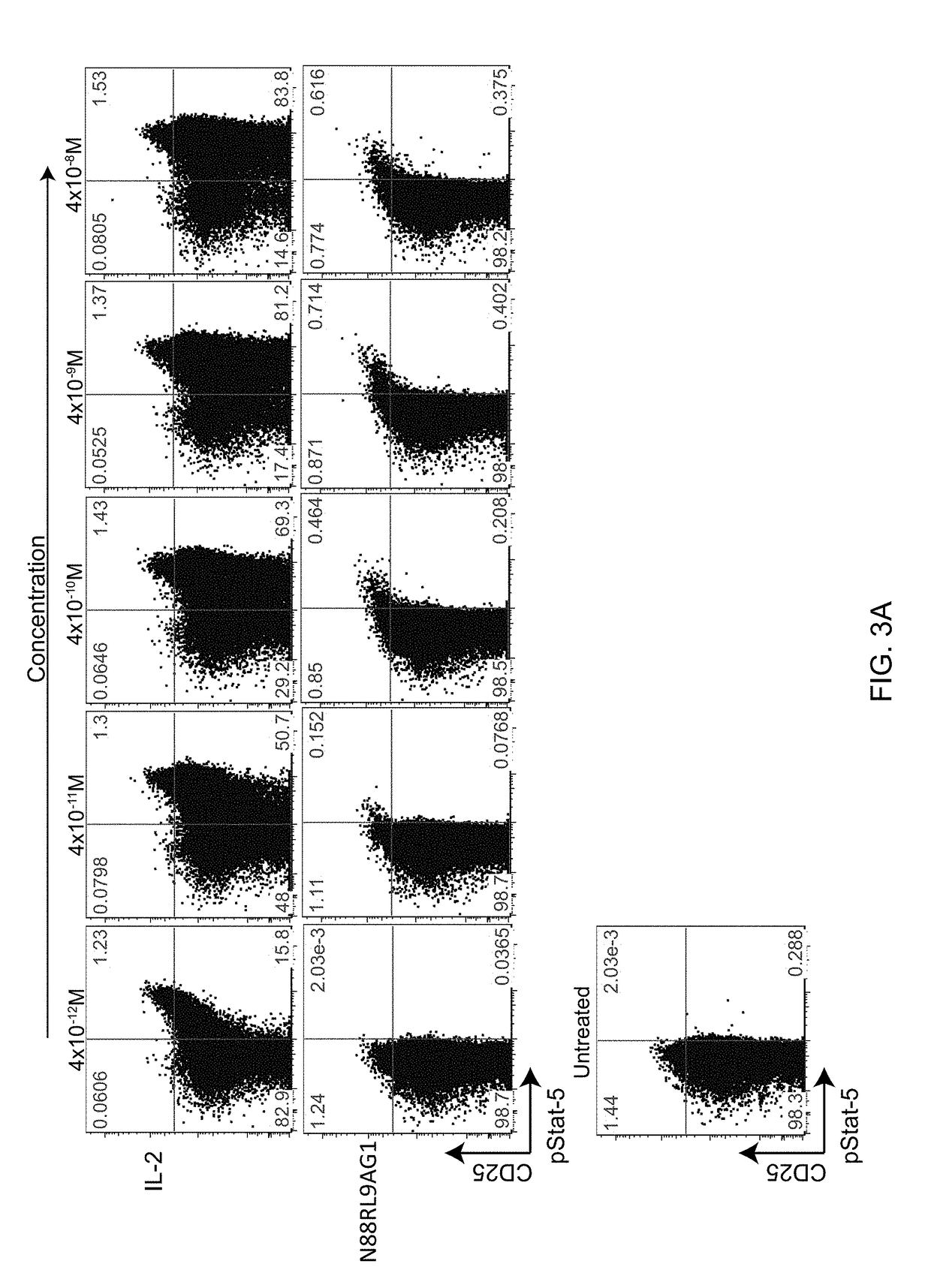
[0096]The bioactivity of N88RL9AG1 and D20HL0G2 on T cells was determined by measuring phosphorylated STAT5 (pSTAT5) levels in specific T cell subsets. Levels of pSTAT5 were measured by flow cytometry in fixed and permeabilized cells using an antibody to a phosphorylated STAT5 peptide. Treg cells constitutively express CD25, and cells that are in the top 1% of CD25 expression levels are highly enriched for Treg cells (Jailwala, P., et al., 2009, PLoS One. 2009; 4:e6527; Long, S. A., et al., 2010, Diabetes 59:407-15). Therefore, the flow cytometry data was gated into CD25high (the top 1-2% of CD25 expressing cells) and CD25− / low groups for the Treg and CD4 effector T cell subsets, respectively.
[0097]Cryopreserved CD4+ T cells (Astarte Biologics, Seattle, Wash.) were defrosted, washed in X-VIVO 15 (Lonza, Allendale, N.J.) media containing 1% human AB serum (Mediatech, Manassas, Va.) and allowed to recover for 2 hours at 37 C. Cells were then dist...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com