Method for preparing hydroxyethyl (METH) acrylate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
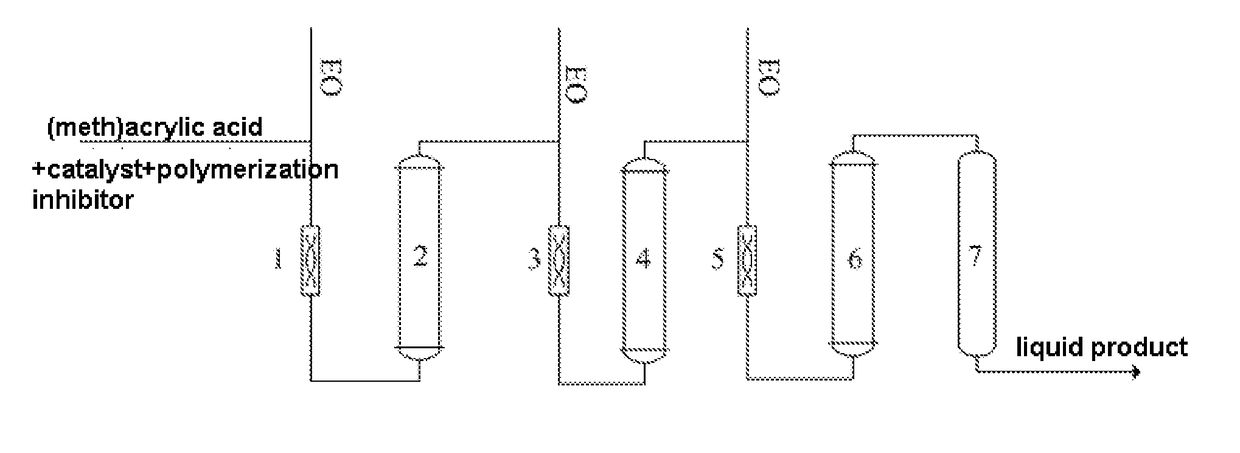
[0033]A process of combining three tubular reactors with an adiabatic tower reactor was adopted, a 316L stainless steel pipe with a inner diameter of 0.02 m was used in the tubular reactors, the length of the first tubular reactor was 0.85 m, the length of the second tubular reactor was 1.40 m, the length of the third tubular reactor was 2 m, the tower reactor was a standard bubbling tower reactor, and the residence time of the materials in the adiabatic reactor was 0.5 h.
[0034]Firstly, 500 kg methacrylic acid, 2.5 kg chromium formate, 0.5 kg 4-hydroxy-2,2,6,6-tetramethyl-piperidinooxy (ZJ-701) were added into a mixing tank and were stirred until the solids were dissolved, and the solution of methacrylic acid raw material was obtained; EO was added respectively into three static mixers; the EO added into the first static mixer and the solution of methacrylic acid raw material were mixed in the first static mixer and then were conveyed to the first tubular reactor (it is called react...
example 2
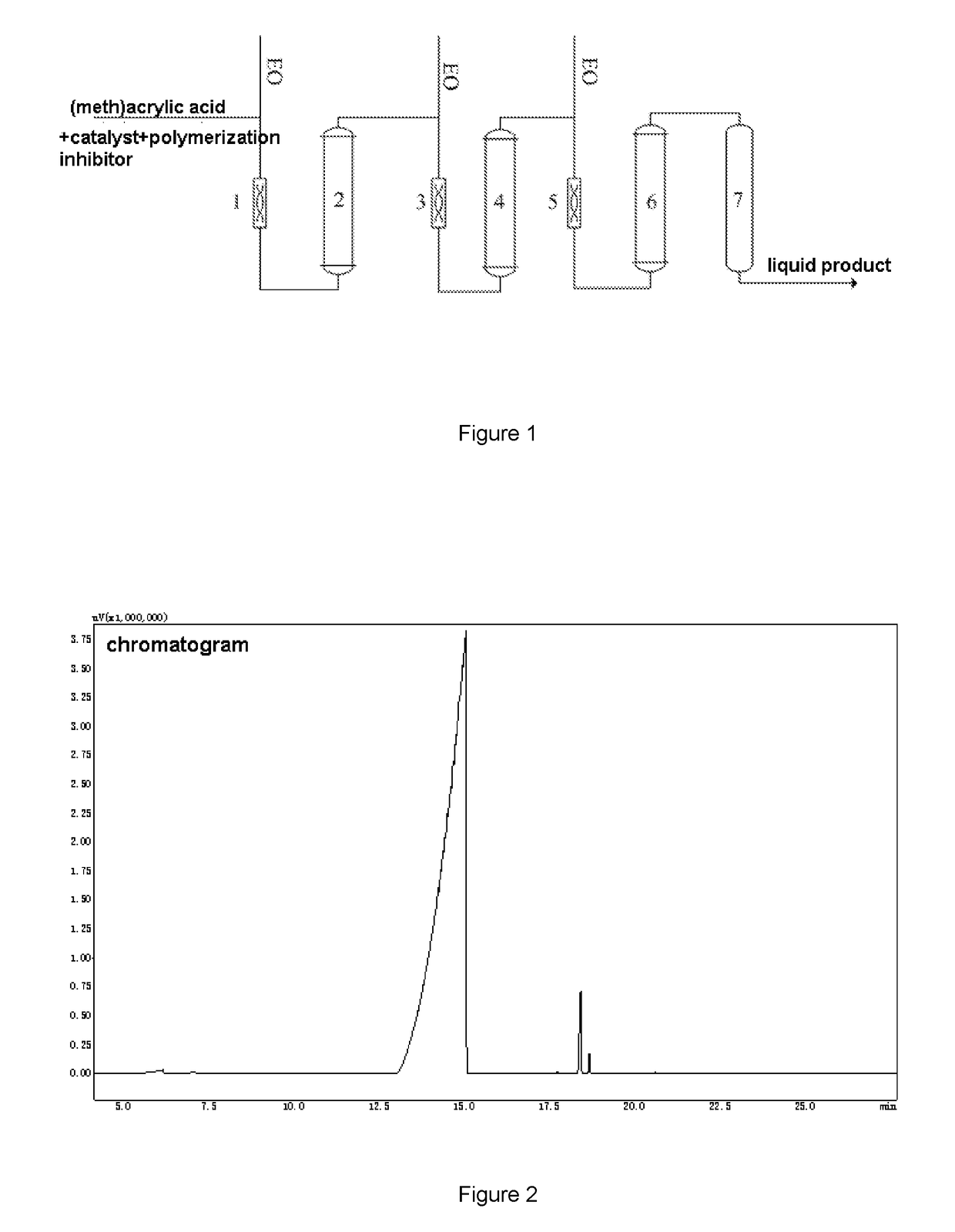
[0035]The difference between example 2 and example 1 lied in that the EO feeding ratios (i.e. the weight ratios of the EO respectively added into the first static mixer, the second static mixer, the third static mixer) was different, the effect of the ratios on the reaction performance was studied, and the other parameters were the same as that in example 1. The products were analyzed by gas chromatography. The results are shown in table 2.
TABLE 2the analysis result of the liquid product of example 2EOfeedingThe composition of the productsratiosMAA / %HEMA / %Monoester / %Diester / %Others / %60:30:100.3696.522.470.210.4450:30:200.5595.952.890.150.4670:20:100.2995.823.010.260.6220:50;300.9694.173.550.570.75
example 3
[0036]The difference between example 3 and example 1 was that the present example provided the influence of different reaction temperatures on the reaction performance. The products were analyzed by gas chromatography. The results are shown in table 3.
TABLE 3the analysis result of the liquid product of example 3TheTheThetemperaturetemperaturetemperatureof reactorof reactorof reactorThe composition of the products1 / ° C.2 / ° C.3 / ° C.MAA / %HEMA / %Monoester / %Diester / %Others / %1001151250.3696.522.470.210.44901101200.6596.152.590.250.361101201300.2995.972.840.360.541201001101.2195.312.750.310.42
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com