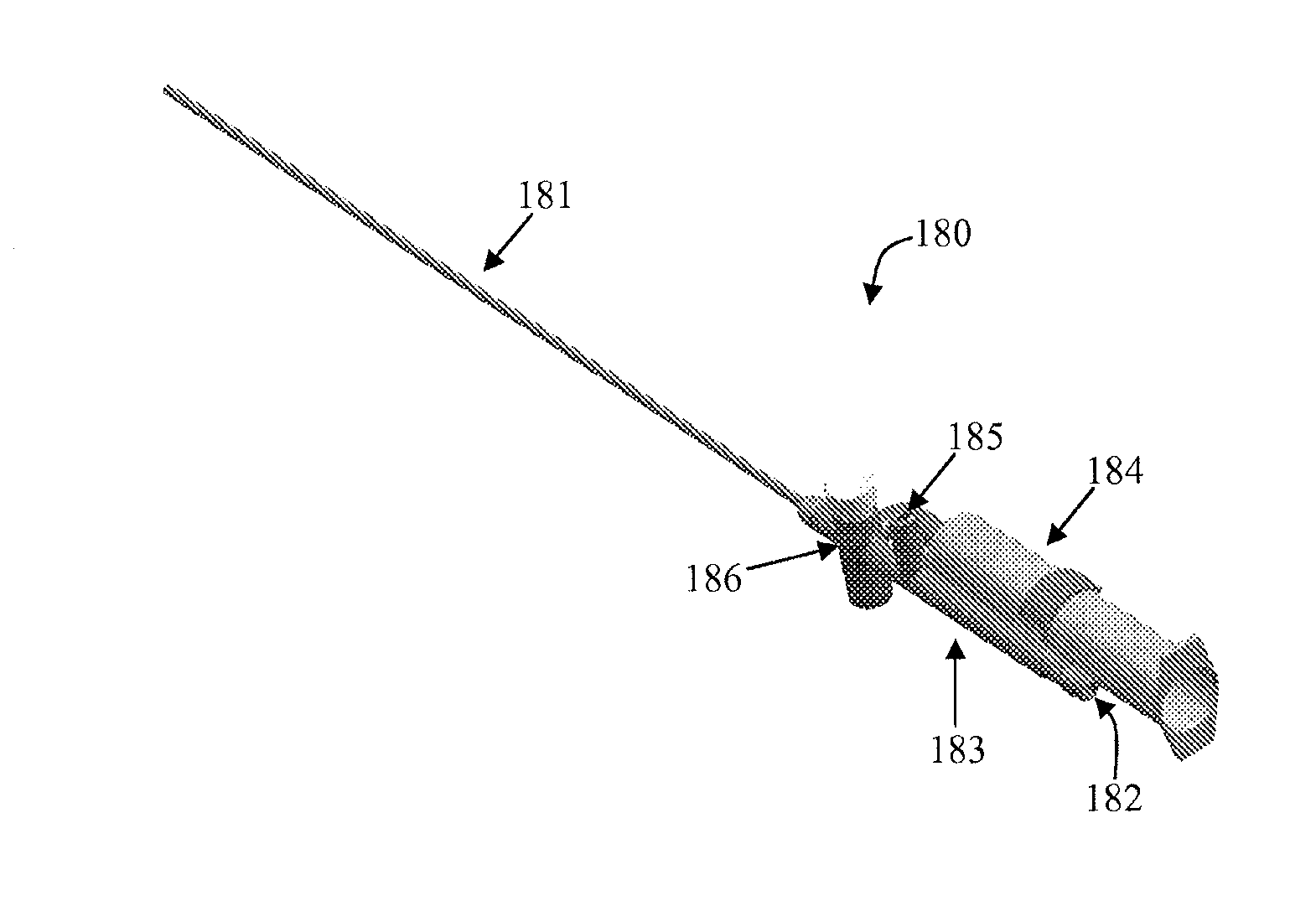
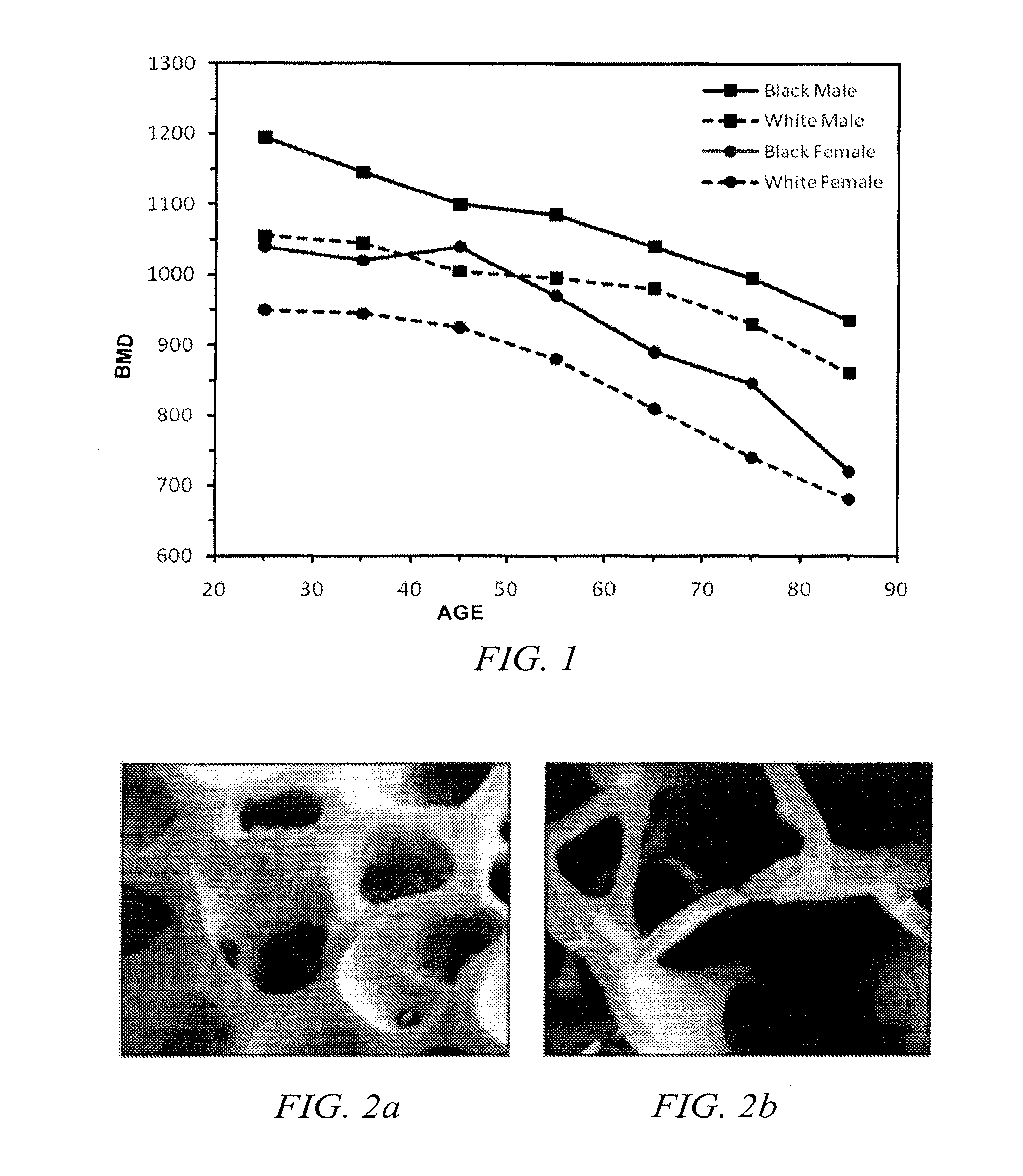
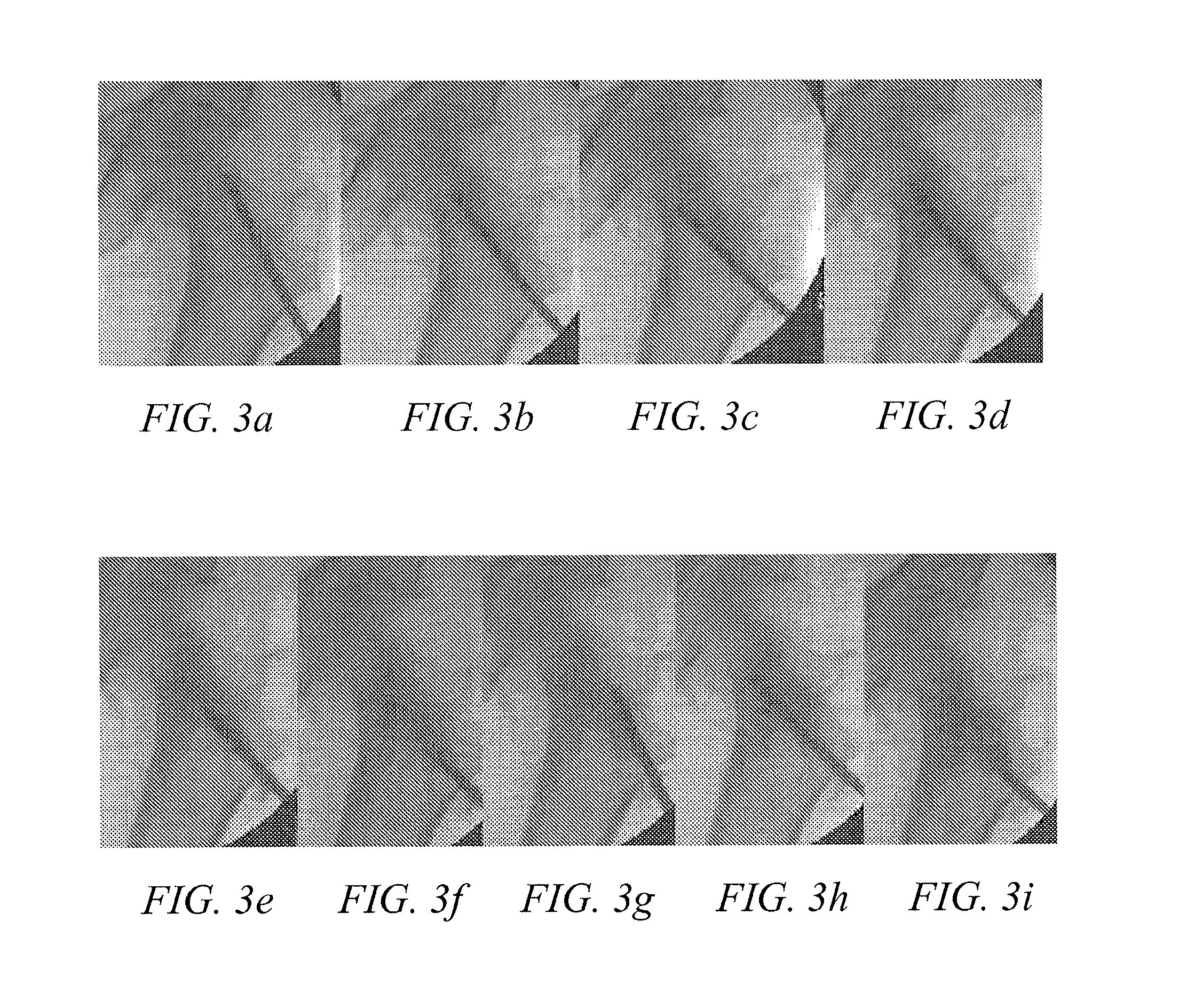
Methods of treating degenerative bone conditions
a bone degeneration and bone technology, applied in the field of bone degeneration treatment methods, can solve the problems of correlated bone fractures (particularly hip or vertebral fractures), the risk of bone fractures is expected to increase, and the ultrasonic is generally limited to the evaluation of the calcaneus bone and is not useful directly, so as to facilitate the carrying out of various inventive methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Resorption Characteristics of Tri-Phasic Bone Regenerative Material
[0175]An accelerated model illustrating the resorption characteristics of a tri-phasic bone regenerative material was carried out using pre-cast and weighed 4.8 mm×3.2 mm pellets of the bone regenerative material that is commercially available under the name PRO-DENSE®. The test was designed to illustrate the changes over time in the bone regenerative material for facilitating controlled in-growth of new bone material. The accelerated in vitro model is approximately six times faster than the resorption seen in vivo in a canine model, and the resorption rate of the in vitro model is even faster in relation to human models.
[0176]To begin the evaluation, the pellets were immersed in distilled water. For daily testing, the pellets were removed from the water, dried, and weighed to determine percent mass remaining. The pellets were placed in fresh aliquots of distilled water after measurements were taken. To analyze micro...
example 2
Comparative Fracture Resistance in Osteoporotic Bone Before and after Void Formation and Filling with Bone Regenerative Material
[0178]To evaluate the effect on fracture susceptibility immediately after performing a procedure according to the invention, cadaver studies were carried out using ten matched pairs of osteopenic or osteoporotic proximal femora. Initial DEXA scans were carried out at the femoral neck and Ward's area, and the T-scores for all tested bones were less than or equal to −2.0, which was indicative of the bone material being in an osteopenic or osteoporotic condition at the time of the testing. The matched pairs were the right and left femur from the same cadaver. In each test, a defect was created in one femur and filled with PRO-DENSE® graft material. The radiographs in FIG. 20 and FIG. 21 show, respectively, insertion of a debridement probe used in creation of the void in the proximal femur and the graft material in place (dark area) filling the formed void. The...
example 3
In Vivo Canine Study Using Bone Regeneration Material in a Large, Critically Sized, Longitudinal Proximal Humerus Model
[0180]A study was carried out to evaluate the I 3 and 26 week in vivo performance of bone regeneration materials in a critically sized canine longitudinal proximal humerus defect model. The biologic response, namely new bone formation, implant degradation, and biocompatibility, were evaluated qualitatively through radio graphs and histology slides.
[0181]In this study, 16 skeletally mature canine subjects each received bilateral longitudinal cylindrical defects (13 mm OD×50 mm) in their proximal humeri. All subjects received OSTEOSET® calcium sulfate bone graft substitute pellets (Wright Medical Technology, Inc., Arlington Tenn.) in one of the two defects. The contralateral defects were treated with either an injected bolus of flowable PRO-DENSE® graft material or preformed pellets of the PRO-DENSE® material, both of which are commercially available. Half of each exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com