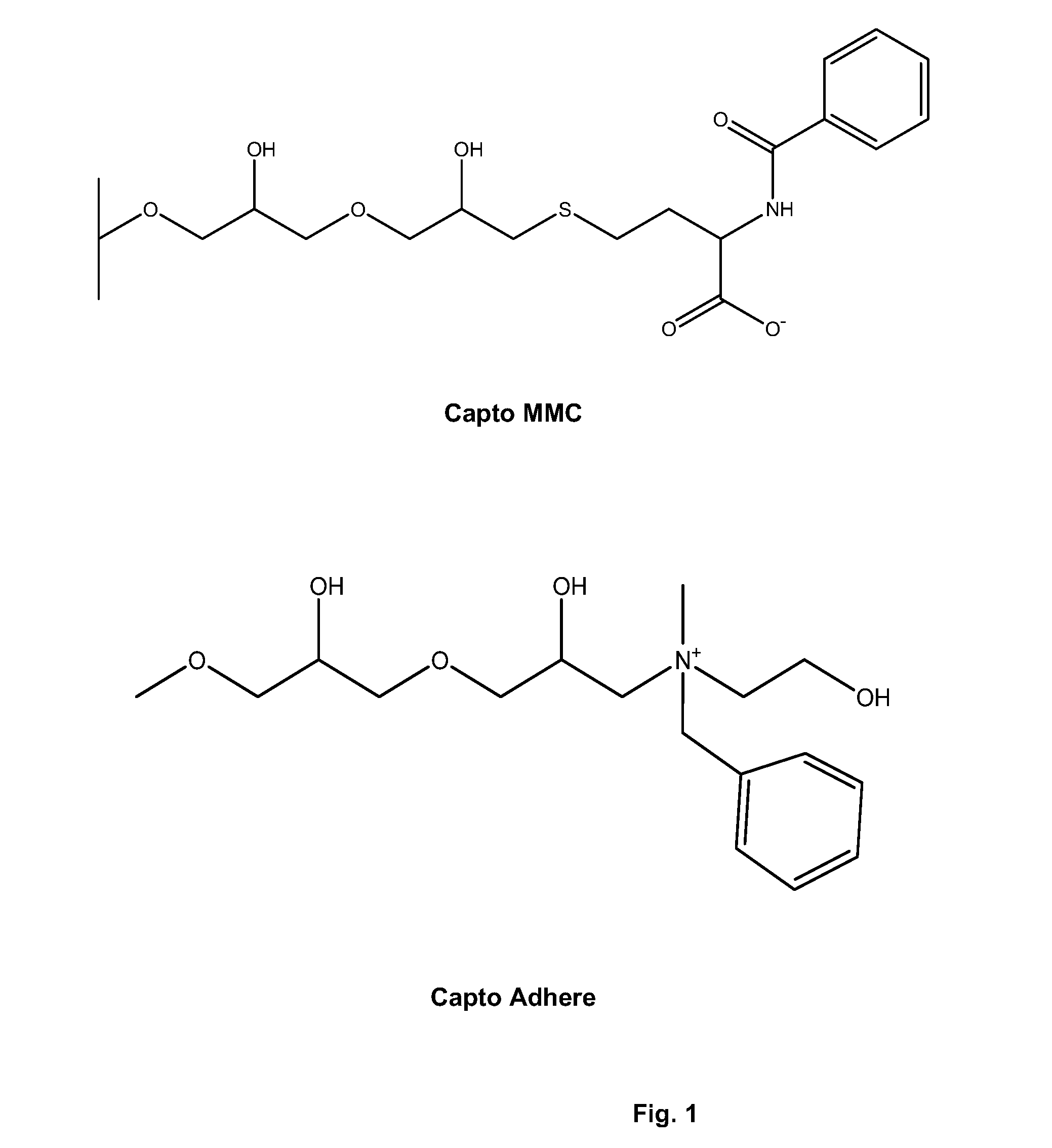
Correctly folded etanercept in high purity and excellent yield
a technology of etanercept and folds, applied in the field of chromatographic separation methods for purifying recombinantly expressed proteins, can solve the problems of large amount of incorrectly or misfolded products produced by cho cells, similar loss of therapeutic effect, and detrimental to patients, so as to maximize the stability and solubility of etanercept, minimize discomfort to patients, and maximize the effect of active ingredient stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Capto™ MMC Mixed Mode Purification Using NaCl Elution
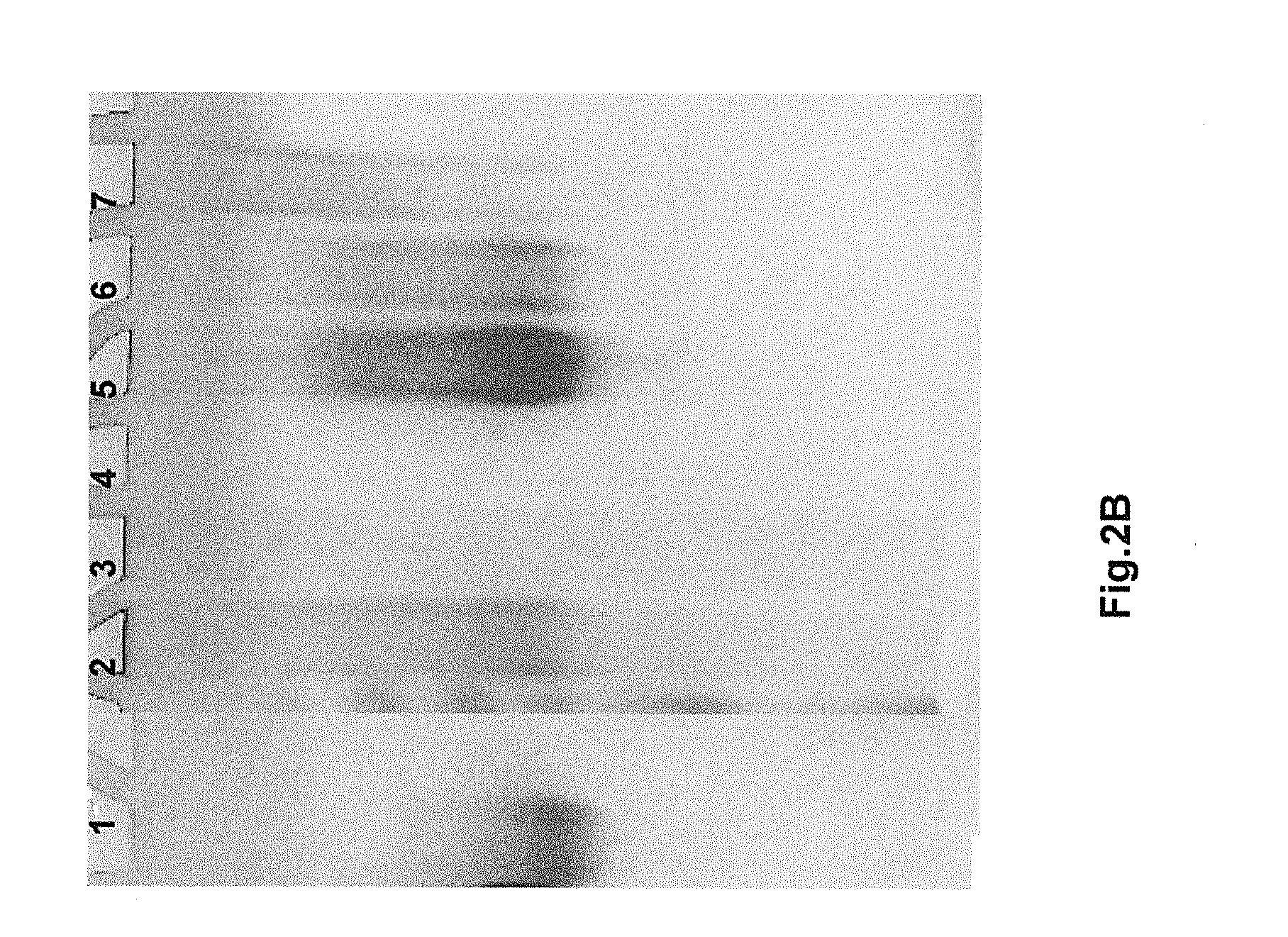
[0139]In this example, a Protein A eluate obtained as described above is subjected to Capto™ MMC chromatography. The Capto™ MMC column is equilibrated with a 5 mM citrate, pH 4.5 solution. An appropriate dilution of Protein A eluate (e.g., 3 to 6 fold dilution depending on the elution buffer of Protein A step) with 5 mM citrate results in complete binding to the column. As was shown in SDS-PAGE analyses, the loading sample (pH 4.2, Protein A eluate) is highly heterogeneous and no protein flows through the Capto™ MMC column. The bound proteins is then eluted with a 0.15 M NaCl in 10 mM phosphate, pH 7.5 solution which leads to a simultaneous change in pH (from 4.5 to 7.5) and NaCl concentration (from 0 to 0.15 M). A sharp elution peak containing correctly folded etanercept is observed from analysis of the eluate. However, the recovery was about 40-50%. Following the elution step as just described, the resin containing the remaining...
example 2
Capto™ MMC Mixed Mode Purification Using NaCl Resin Equilibrated to pH 7.5
[0140]In the foregoing example, the protein solution comprising correctly folded and incorrectly folded etanercept was initially contacted with the CAPTO MMC resin at pH 4.5. In this example, a similar elution to that observed in Example 1 is observed when the Capto™ MMC column is first equilibrated with a 10 mM phosphate, pH 7.5 solution and then eluted with NaCl followed by arginine. Specifically, no etanercept is eluted during this pH equilibration. This result is unexpected because etanercept is negatively charged at pH 7.5; the pI of etanercept ranges from 4.9 to 5.4 due to heavy glycosylation. Thus, at or below pH 4.5, etanercept is positively charged and hence should bind to the negatively charged Capto™ MMC ligands, although hydrophobic interaction may contribute to the binding. At pH 7.5, the negatively charged etanercept should dissociate from the negatively charged Capto™ MMC, but it is found that t...
example 3
Capto™ MMC Mixed Mode Purification Using NaCl Gradient for Elution
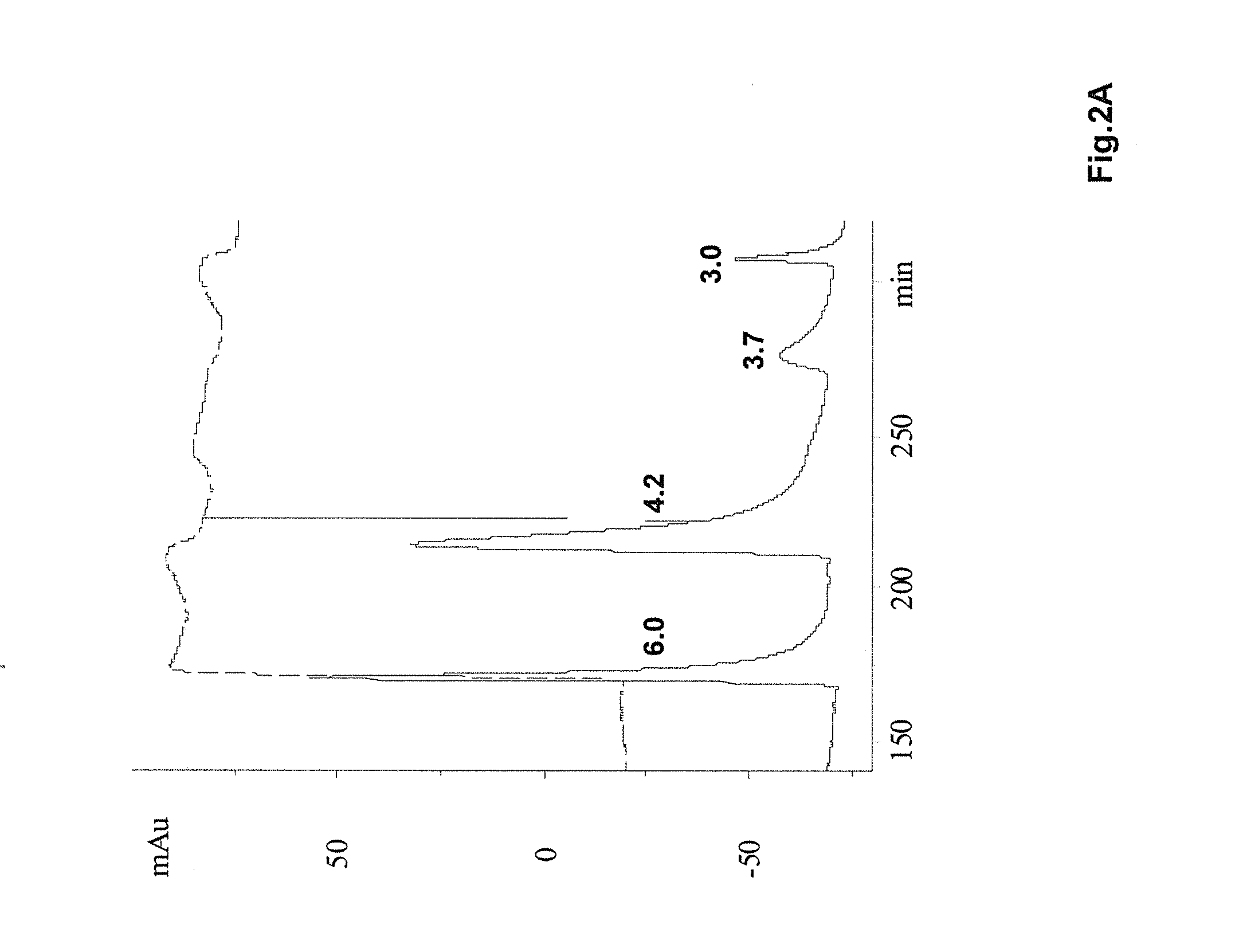
[0141]In this example, successful elution is accomplished using an NaCl concentration gradient. Specifically, after washing the column with the 10 mM phosphate, pH 7.5 solution, the bound proteins were eluted with linear salt gradient from 0 to 0.5 M. The loading sample (pH 4.2 Protein A eluate) is extremely heterogeneous (containing both correctly folded and incorrectly folded etanercept) as in the previous case, and the bound proteins are eluted with increasing ionic strength of the NaCl gradient. After 180 min, the gradient is terminated and salt concentration is then brought to 0.5 M, which caused a slightly enhanced protein elution. As determined by subsequent analyses, low salt fractions contain more of the correctly folded etanercept and higher salt fractions were enriched with low mobility (misfolded and aggregated) species. Finally, the remaining proteins is eluted with a 1 M arginine, 10 mM phosphate, pH 7.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com