Novel composition for tumor growth control
a tumor growth and composition technology, applied in the field of cancer, can solve the problems of dna methyltransferase making mistakes, interfering with the binding of transcription factors, and de novo methylation of cpg islands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0043]Methods
[0044]Cell Lines and Culture Conditions
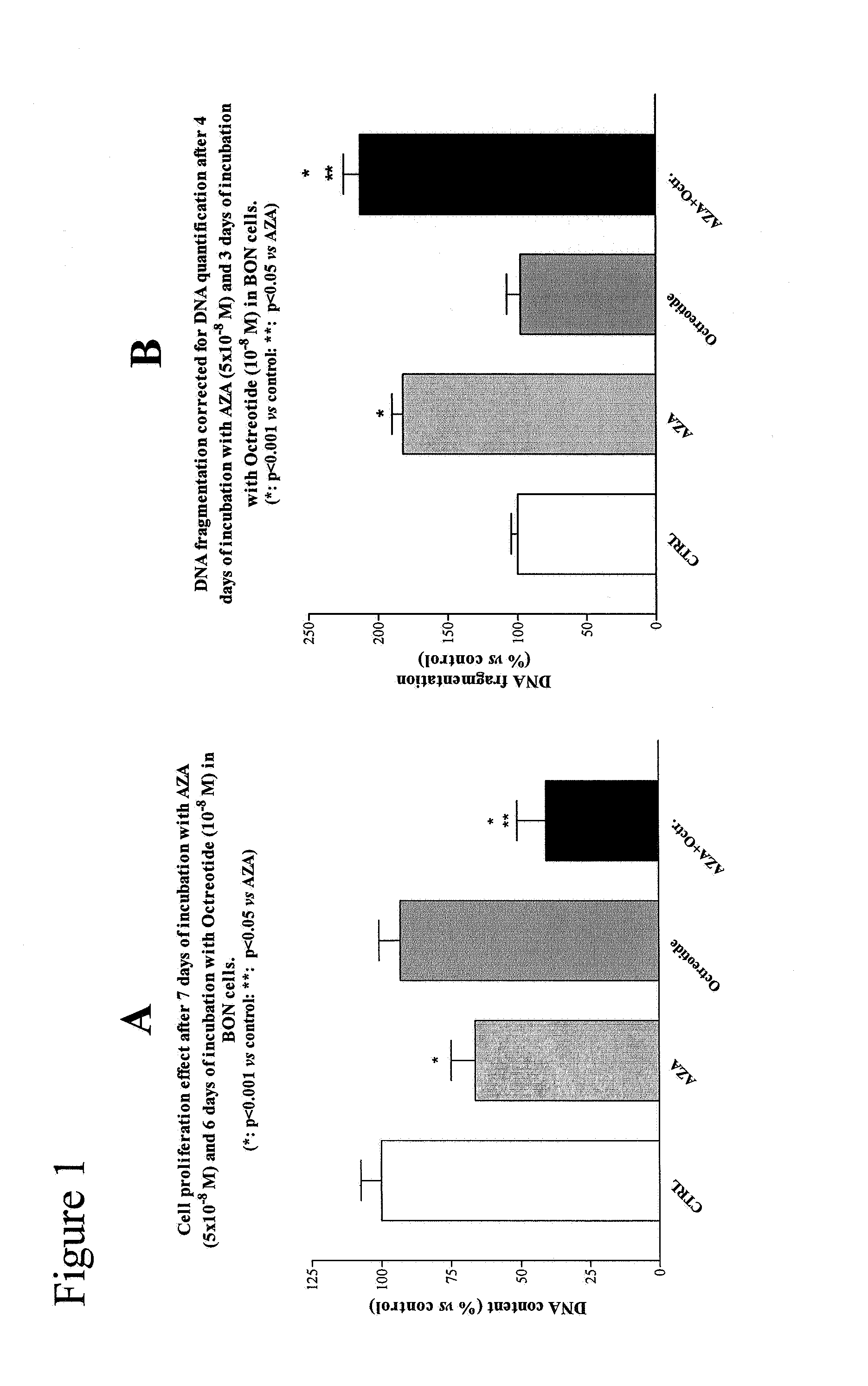
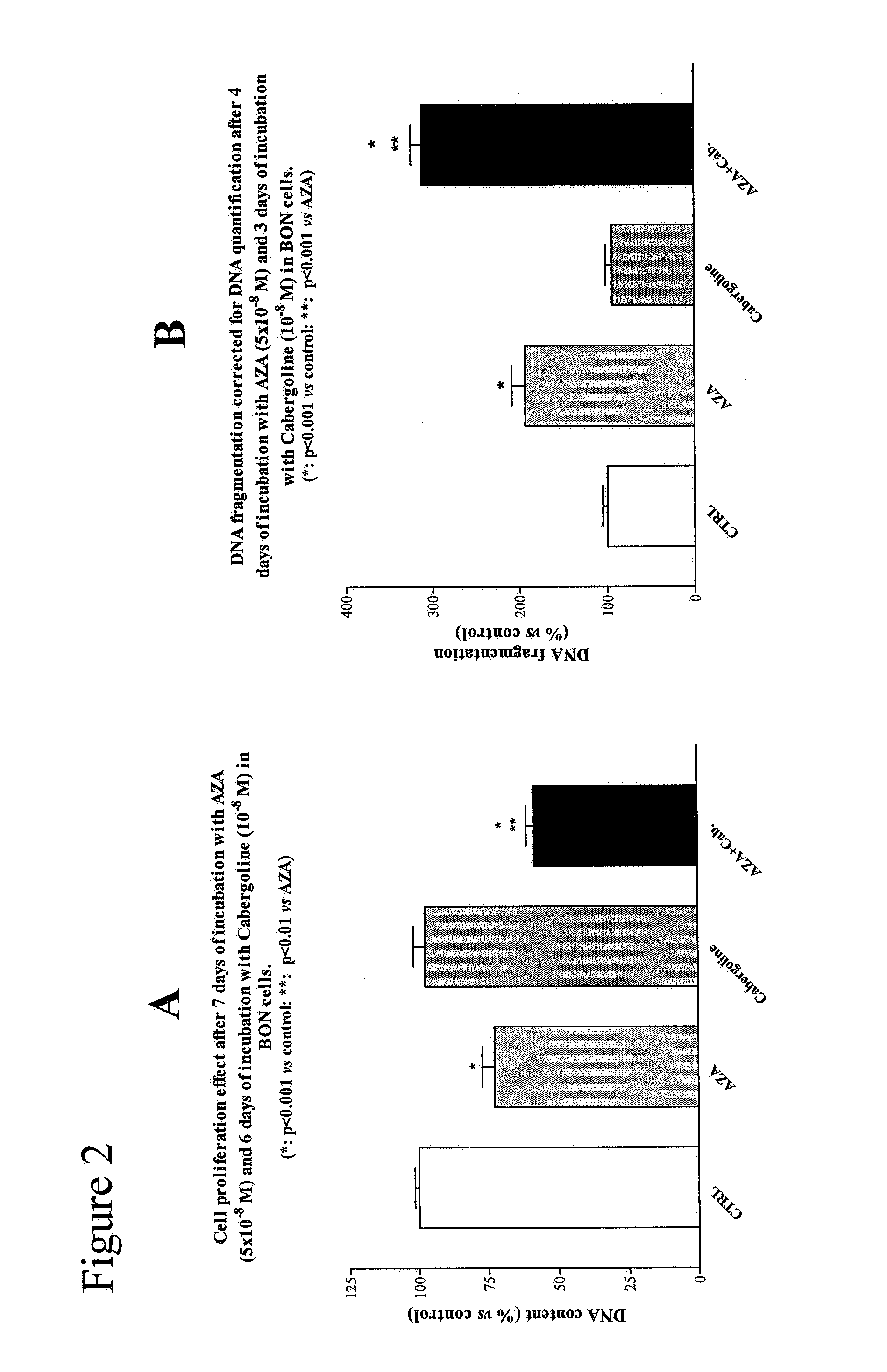
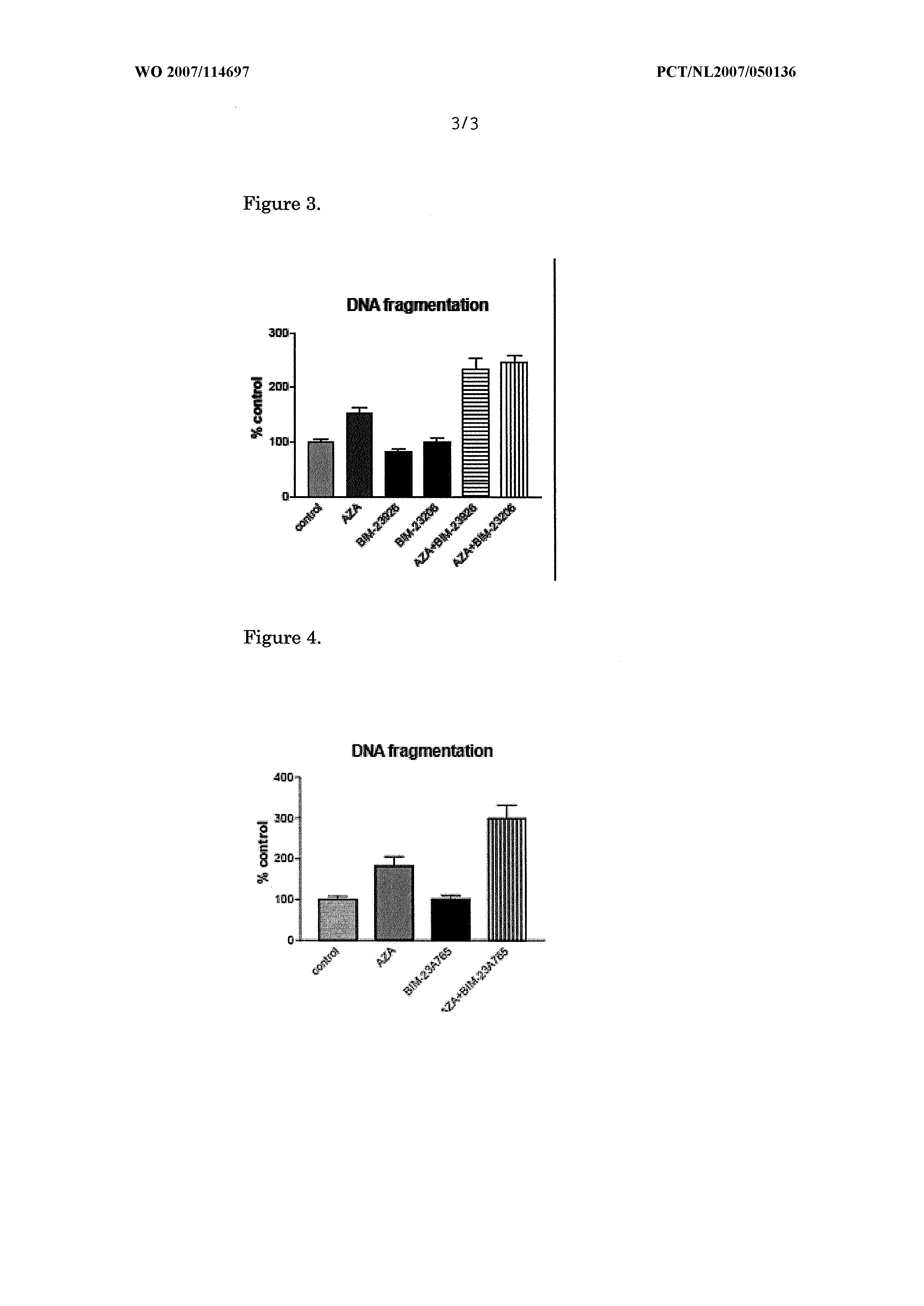
[0045]Human pancreatic carcinoid BON cells were obtained from Dr Townsend (The University of Texas Medical Branch, Galveston, Tex., USA). The cells were cultured in a humidified incubator containing 5% CO2 at 37° C. The culture medium consisted of a 1:1 mixture of Dulbecco's modified Eagle's medium (DMEM) and F12K medium, supplemented with 10% FCS, penicillin (1×105 U / l), fungizone (0.5 mg / l) and 1-glutamine (2 mmol / l). Periodically, cells were confirmed as Mycoplasma-free. Cells were harvested with trypsin EDTA 10% and resuspended in medium. Before plating, cells were counted microscopically using a standard hemocytometer. Trypan Blue staining was used to assess cell viability, which always exceeded 95%. Media and supplements were obtained from GIBCO Bio-cult Europe (Invitrogen, Breda, The Netherlands).
[0046]Cell Proliferation Assay
[0047]After trypsinization, the cells were plated in 1 ml of medium in 24-well plates at a density o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com