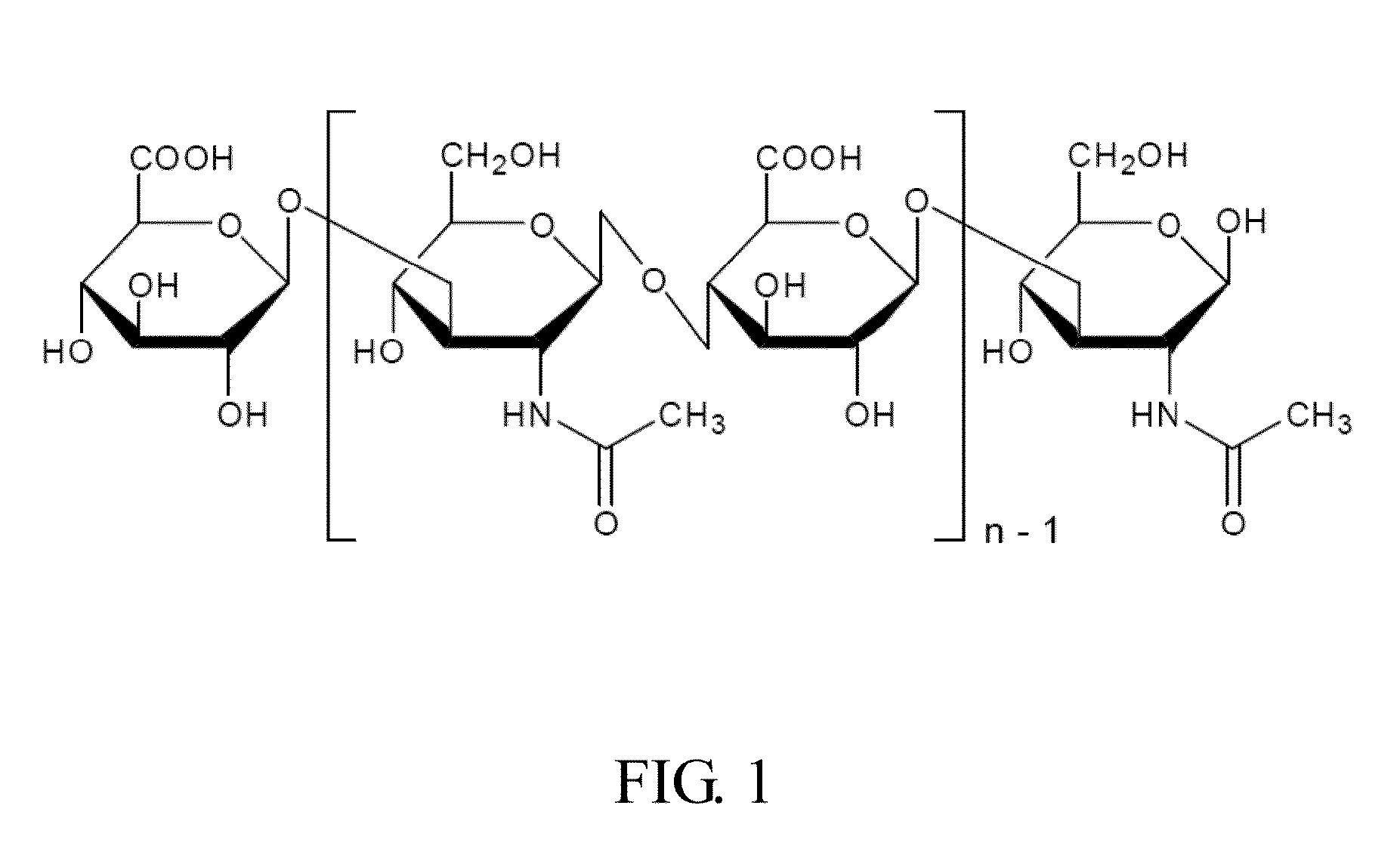
Materials for treating and preventing mucosa related disease
a technology of hyaluronic acid and mucosa, which is applied in the field of composition of hyaluronic acid, can solve the problems of inconvenient clinical treatment for patients, lack of quick and long-term effects, and inability to use ha species with certain average molecular weight for prompt treatment and sustained
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
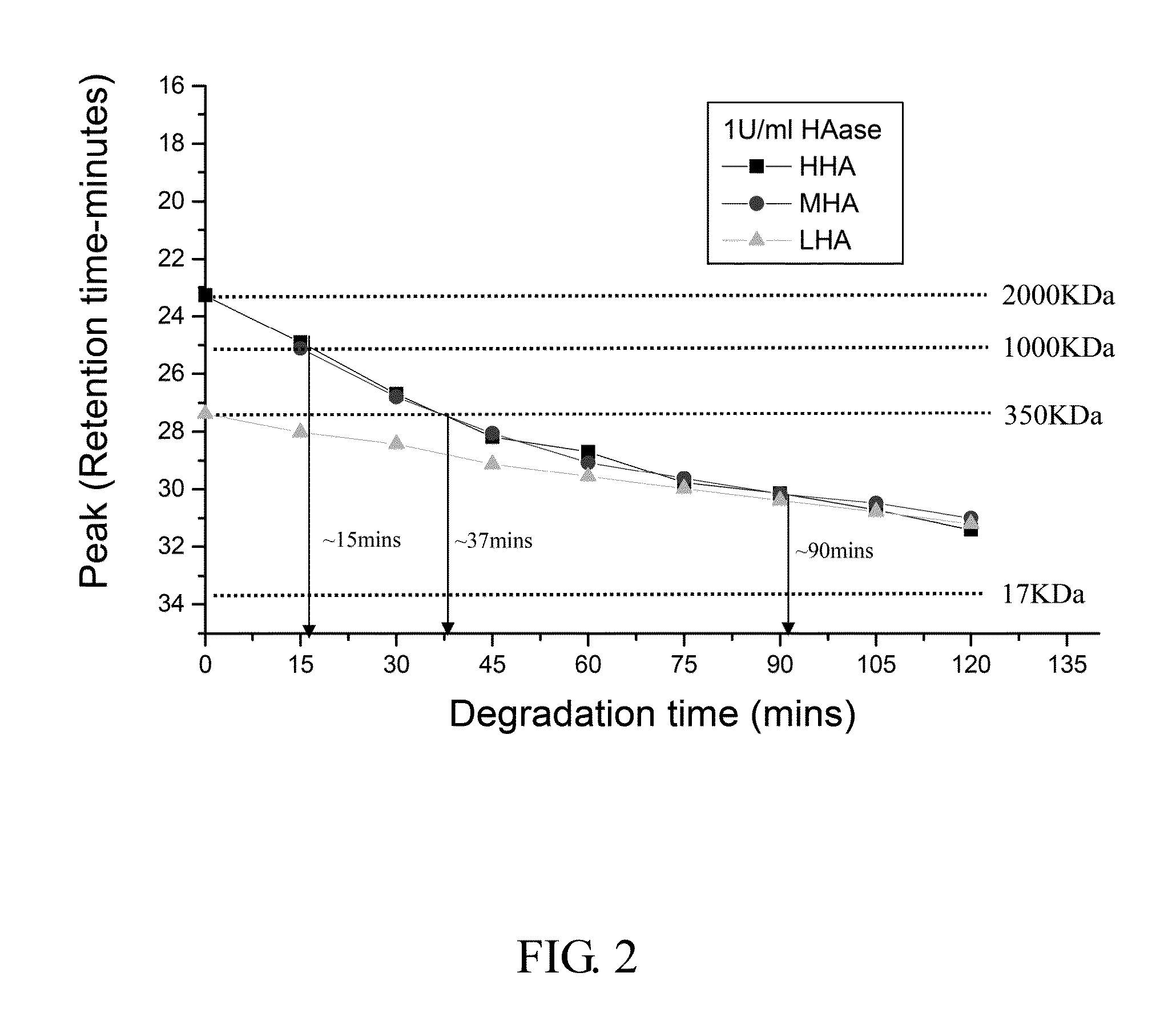
example 1
The Degradation of HA in 1 U / ml HAase
[0064]Procedure:
[0065]1. 0.25 g High molecule weight sodium hyaluronate powder (HHA; Mw: 2 MDa; Freda) and 0.25 g low molecule weight sodium hyaluronate powder (LHA; Mw: 0.35 MDa; Freda) were added into 50 ml PBS buffer (Phosphate buffered saline) respectively to form 0.5% solution, and then stirred for 6 hours until the powder was totally dissolved.
[0066]2. 0.05 g LHA powder and 0.2 g HHA powder (ratio 2:8; medium molecular weight sodium hyaluronate powder, MHA) were added into 50 ml PBS buffer, and then stirred for 6 hours until the powder was totally dissolved.
[0067]3. Mobile phase solution of GPC (Gel permeation chromatography) system was prepared by: (1) adding 35.49 g Na2HPO4 powder into 450 ml deionized distilled water (dd water) and stirred for 30 minutes in room temperature to form 0.5 M Na2HPO4 solution; and (2) adding 18 g NaH2PO4 powder into 250 ml dd water and stirred for 30 minutes in room temperature to form 0.5 M NaH2PO4 solution....
example 2
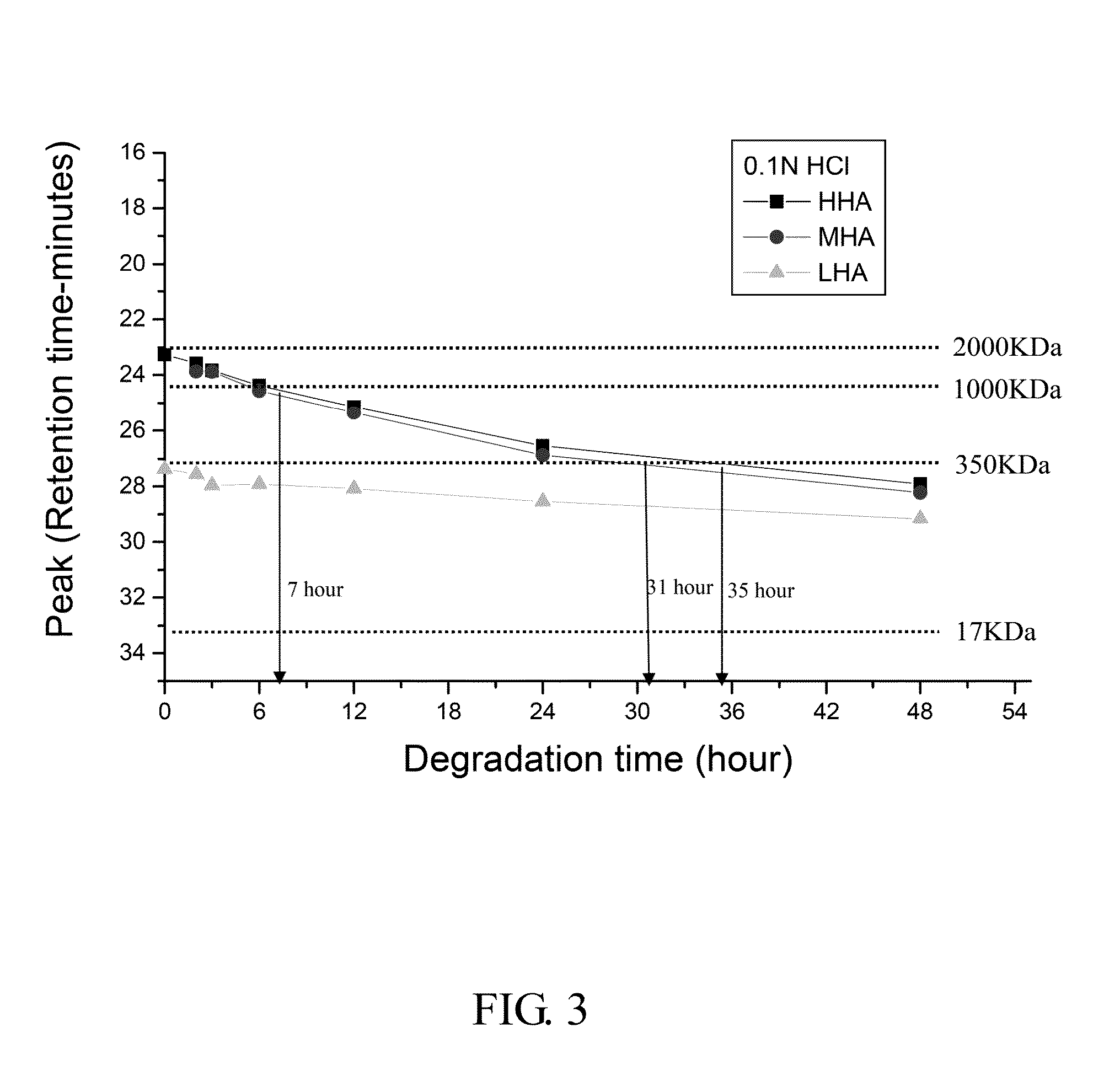
The Degradation of HA in 0.1 N HCl
[0075]Procedure:
[0076]1. LHA, MHA and HHA were prepared as the same as Example 1.
[0077]2. Mobile phase solution of GPC system was prepared as the same as Example 1.
[0078]3. Artificial gastric juice (0.1 N HCl) was prepared by mixing 5.72 ml 17.5 N HCl and 90 ml dd water and stirred for 10 minutes as a stocking solution.
[0079]4. 2 ml of HHA, MHA and LHA were mixed with 8 ml artificial gastric juice, respectively in a 15 ml glass tube and by vortex for 3 minutes.
[0080]5. The tube was shaken by 50 rpm in 37° C. water bath. 1 ml solution was taken after the 6, 12, 24, 48 hours and then supplied with 1 ml artificial gastric juice each time. Every 1 ml solution was filtered through 0.45 μm filter. 20 μl solution was injected into GPC system and then the diagram was recorded.
[0081]6. All values in the table were expressed as means of n observations. The histological index was analyzed by Student's t-test.
[0082]Result:
[0083]FIG. 3 shows the retention time o...
example 3
The Adhesion of HA in Colon Tissue (IVIS Image System-Vision 3)
[0084]Procedure:
[0085]1. LHA and HHA were prepared as the same as Example 1. MHA (MHA; Mw: 1 MDa; Freda) were added into 50 ml PBS buffer, and then stirred for 6 hours until the powder was totally dissolved and ready for use in the following steps.
[0086]2. Fluorescent HA (HA-f) was prepared by (1) 0.39 g MES free acid (2-(N-morpholino) ethanesulfonic acid, Calbiochem) and was dissolved in 100 ml dd water. (2) Solution A: 65 mg fluororesceinamine powder, (isomer I, Fluka) was dissolved in 9 ml 95% EtOH solution and then stirred for 10 minutes under a condition that light was prohibited. (3) Solution B: 359 mg EDC powder (N-(3-Dimethylamino propyl)-N-ethyl carbodiimide hydrochloride, Sigma) was dissolved in 9 ml MES buffer and then stirred for 10 minutes. (4) Solution C: 216 mg NHS powder (N-Hydroxysuccinimde, Sigma) was dissolved in 9 ml MES buffer and then stirred for 10 minutes. (5) 3 ml Solution A was slowly dropped in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mw | aaaaa | aaaaa |
| Mw | aaaaa | aaaaa |
| Mw | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com