Biphasic lipid-vesicle composition and method for treating cervical dysplasia by intravaginal delivery
a lipid-vesicle and composition technology, applied in the direction of peptide/protein ingredients, organic active ingredients, pharmaceutical non-active ingredients, etc., can solve the problems of examining the hpv status post, unable to immediately treat women with hpv, and undergone such treatment options may carry an increased risk of abortion and premature labor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
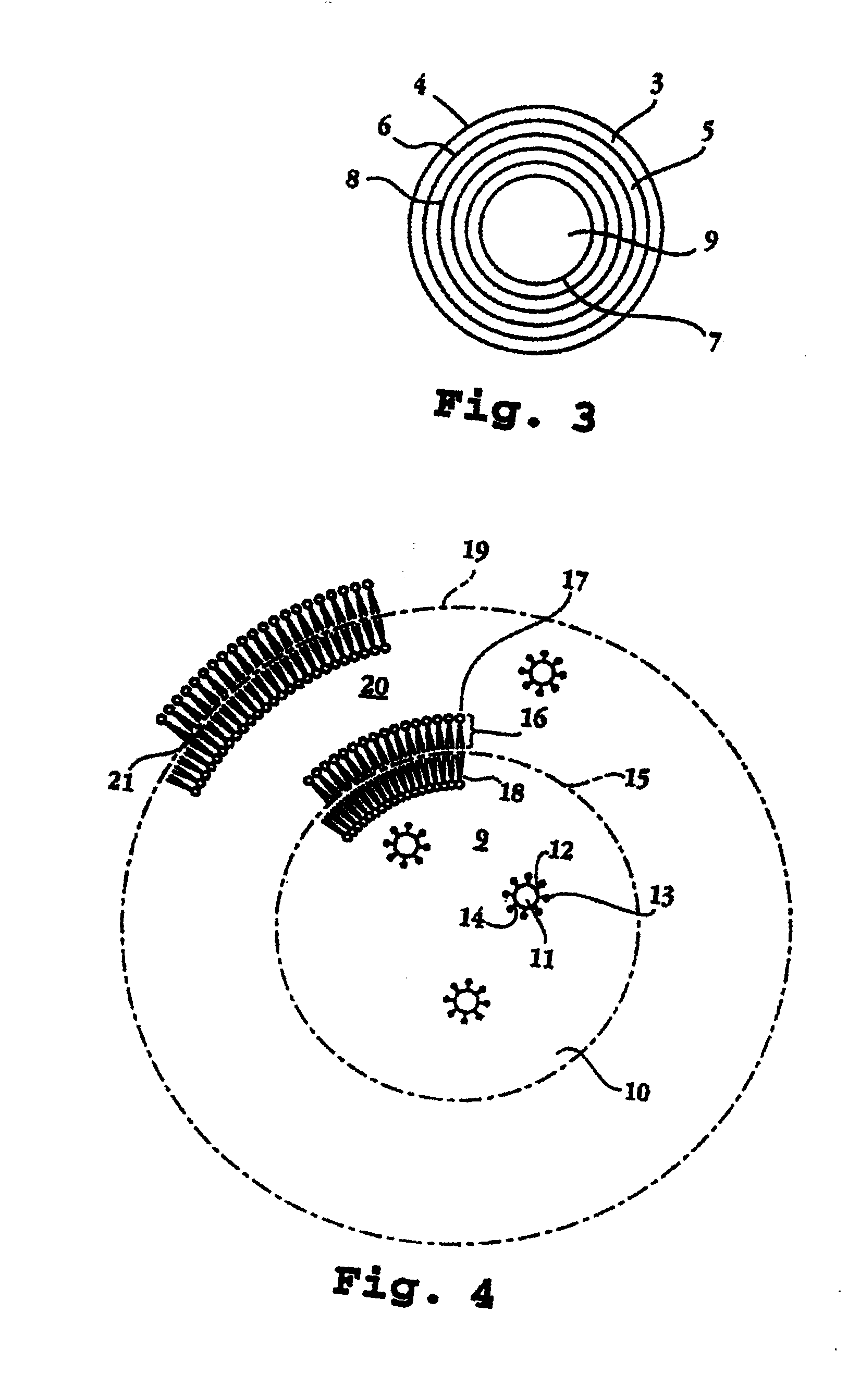
I. Biphasic Liposome Composition and Method of its Preparation
[0021]The invention relates to a lipid-bilayer or liposome or lipid vesicle composition for use in delivering an interferon, e.g., interferon alpha-2b by transmucosal delivery, e.g., by intravaginal administration, particularly in the treatment of cervical dysplasia.
[0022]A preferred method of preparing a multilamellar lipid vesicle of the invention is as follows. An oil and a consistency enhancer are admixed. Separately, water and a surfactant are admixed. A water-soluble antimicrobial agent, for example methyl paraben or propylparaben, a buffering agent, such as phosphates, and a chelating agent, such as EDTA, can also be dissolved in the water. These are heated gently, say to about 70° C., and then admixed and homogenized with the oil and consistency enhancer. This results in formation of an emulsion with water as the continuous phase and the oil and consistency enhancer as the dispersed phase. It is desirable that the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com