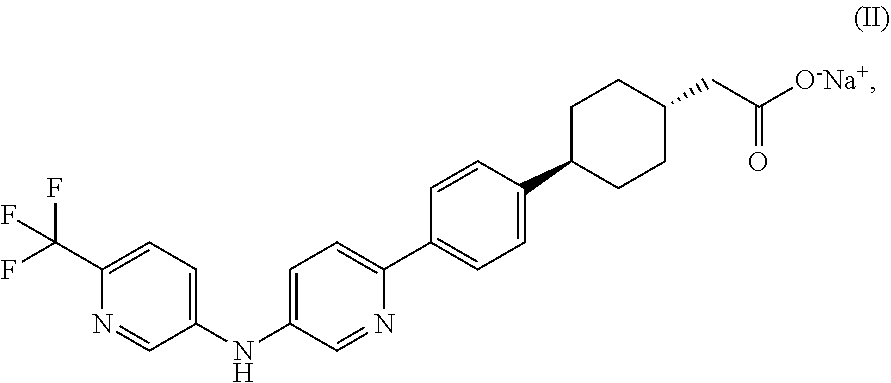
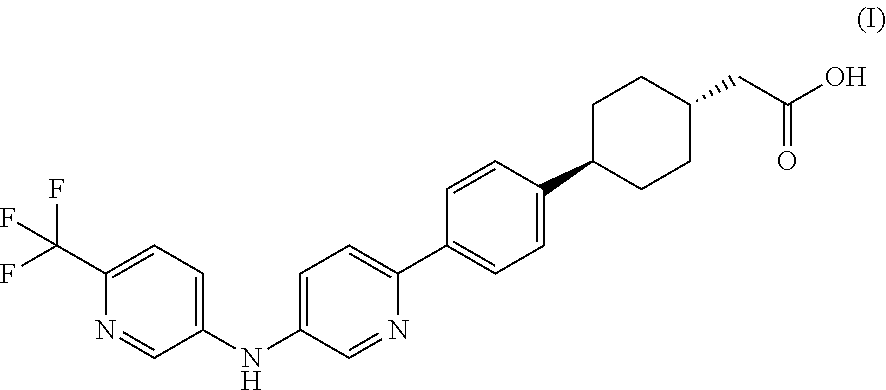
Pharmaceutical compositions containing a dgat1 inhibitor
a technology of dgat1 and composition, which is applied in the field of pharmaceutical compositions, can solve the problems of poor flow characteristics, difficult formulation of drug substances, and fluffy sticky substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 2
[0047]A pharmaceutical composition, according to embodiment 1, wherein the surfactant with lubricant properties is selected from, sodium lauryl sulfate (SLS), stearic acid, palmitic acid, myristic acid, poloxamers and polyethylene glycols such as PEG 4000-8000, Tween series of surfactants. Brij series of surfactants (i.e., Brij 80), Triton X-100, and combinations thereof, preferably Sodium lauryl sulfate (SLS).
embodiment 3
[0048]A pharmaceutical composition, according to embodiment 1 or 2, wherein the dry binder is selected from polyethylene glycols (PEG), e.g., PEG 4000; pregelatinized starch; starch; chitosan; guar gum, microcrystalline cellulose: methyl cellulose; calcium carboxymethylcellulose; sodium carboxymethylcellulose, alginic acid and / or its sodium salt; hydroxypropylmethyl cellulose or hydroxypropyl cellulose, both preferably of medium to high viscosity, e.g., viscosity grades 3 or 6 cps, e.g. low substituted hydroxypropyl cellulose (L-HPC LH-21); and combinations thereof, most preferably, low substituted hydroxypropyl cellulose (L-HPC LH-21).
embodiment 4
[0049]A pharmaceutical composition, according to any one of embodiments 1 to 3, wherein the filler is selected from microcrystalline cellulose (e.g., cellulose MK GR and products available under the registered trade marks AVICEL, FILTRAK, HEWETEN or PHARMACEL, Vivapur, emcocel, tabulose), low-substituted hydroxypropyl cellulose, hydroxyethyl cellulose, hydroxypropyl methyl cellulose, Anhydrous Dicalcium Phosphate, Dicalcium Phosphate, lactose, Anhydrous Lactose, and combinations thereof.
PUM
| Property | Measurement | Unit |
|---|---|---|
| forces | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com