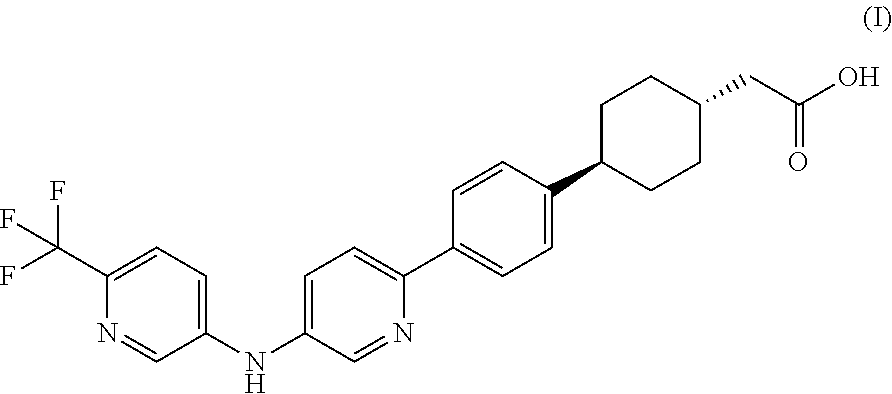
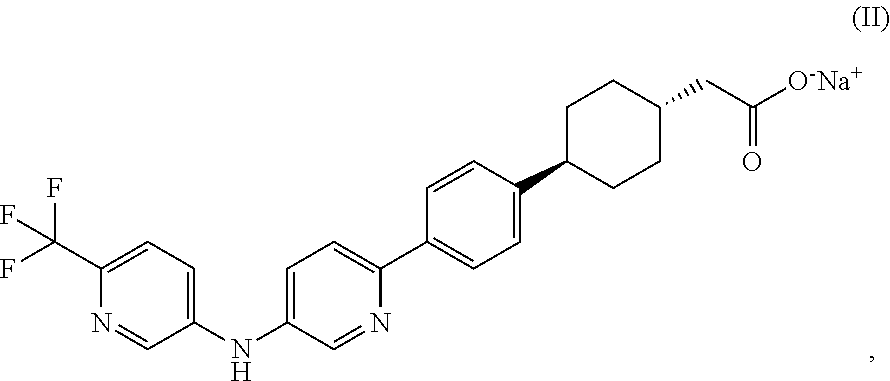
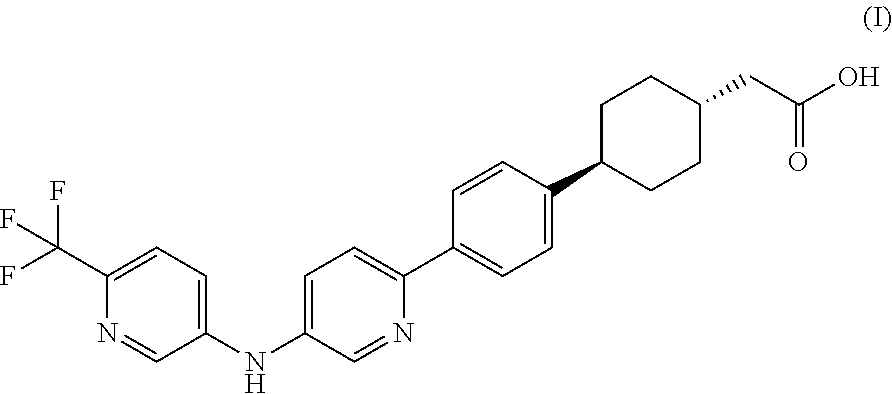
Pharmaceutical compositions containing a dgat1 inhibitor
a technology of dgat1 and composition, which is applied in the field of pharmaceutical compositions, can solve the problems of poor flow characteristics, difficult formulation of drug substances, and fluffy sticky substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Methodology Example A
Dissolution Testing
[0087]The tablets of the Examples are tested for their dissolution in 900 ml of pH 6.8 phosphate buffer with paddles at 75 rpm.
[0088]The assembly consists of the following: a covered vessel made of glass or other inert, transparent material; a motor, and a paddle formed from a blade and shaft as the stirring element. The vessel is partially immersed in a suitable water bath of any convenient size or placed in a heating jacket. The water bath or heating jacket permits holding the temperature inside the vessels at 37±0.5° during the test and keeping the bath fluid in constant, smooth motion. No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation, or vibration beyond that due to the smoothly rotating stirring element. Apparatus that permits observation of the specimen and stirring element during the test is has the following dimensions and capacities: the height is 160 mm to 2...
example b
Methodology Example B
Hardness Testing
[0090]A Schleuniger 8M Hardness tester was used to perform tablet hardness testing. Tablets were positioned on the instrument stage. Each tablet was oriented in the lengthwise same position according to distinguishing marks (when applicable). Testing was performed for 10 tablets from each batch and each compression force.
Example 1
Reference Example
[0091]trans-(4-{4-[5-(6-Trifluoromethyl-pyridin-3-ylamino)-pyridin-2-yl]-phenyl}-cyclohexyl)-acetic acid, sodium salt along with Microcrystalline Cellulose (partial), and Crospovidone (intragranular) are mixed in a low shear mixer. The mixed contents, along with remaining Microcrystalline Cellulose are passed through an oscillating mill equipped with a suitable screen. The screened contents are mixed in a low shear mixer for a suitable amount of time. Colloidal silicon dioxide, screened through an appropriate screen is mixed with the blend from earlier step and the contents are mixed for a suitable amoun...
example 1
Uncoated Tablet Comprising a DGAT1 Inhibitor, (10 mg of Active Ingredient, Based on Free Acid of Compound 1)
[0093]
Ingredientsmg / tabtrans-(4-{4-[5-(6-Trifluoromethyl-pyridin-10.513-ylamino)-pyridin-2-yl]-phenyl}-cyclohexyl)-acetic acid, sodium saltMicrocrystalline Cellulose172.49Crospovidone14.0Colloidal silicon dioxide1.0Magnesium Stearate1.0Total weight100 mg
[0094]The Table below shows the dissolution of tablets of Example 1.A which are compressed at two different hardness i.e. 6 kN and 12 kN. The dissolution for the batches was performed using USP-2 Paddle / 0.4% CTAB / pH 6.8 buffer / 50 rpm.
TABLEDissolution summary of Example 1.A (at two hardness levels)% compound of formulat (II) released (% w / w)Compressionat the following time points (in minutes)hardness10 min20 min30 min45 min60 min 6 kN65829110110312 kN4877828689
PUM
| Property | Measurement | Unit |
|---|---|---|
| forces | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com