METHODS OF ADMINISTERING ANTI-TNFalpha ANTIBODIES
a technology of antitnfalpha and antibody, applied in the direction of drug composition, immunological disorders, metabolism disorders, etc., can solve the problems of short serum half life, inability to trigger certain human effector functions, limited use in vivo, etc., to reduce the number of injection site tractions, less frequent, and increase patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Treatment with an Anti-TNFα Antibody
D2E7 Efficacy Following Subcutaneous Administration
[0123]In this study, twenty-four patients with active RA were treated with weekly doses of 0.5 mg / kg D2E7 (n=18) or placebo (n=6) by s.c. injection for three months. Patients participating in this study had a mean duration of disease of 10.1 years with a disease activity scorn (DAS) scorn of 4.87 and a mean of 3.4 DMARDs (disease modifying anti-rheumatic drugs) prior to study entry; again reflecting considerable disease activity, Responders continued open-label treatment with D2E7, while patients who Palled to respond to the 0.5 mg / kg dose or who lost a DAS response on the 0.5 mg / kg dose were escalated to receive 1 mg / kg by s.c. injection after week twelve of the study.
[0124]The first patients enrolled received up to sixty injections and were, therefore, sixty weeks on the study drug. The efficacy with s.c. dosing was similar to i.v. injections. Up to 78% of patients reached a DAS and ACR20 respon...
example 2
Total Body Dose of a Subcutaneously Administered Anti-TNFα Antibody
Weekly, Subcutaneous Administration of D2E7
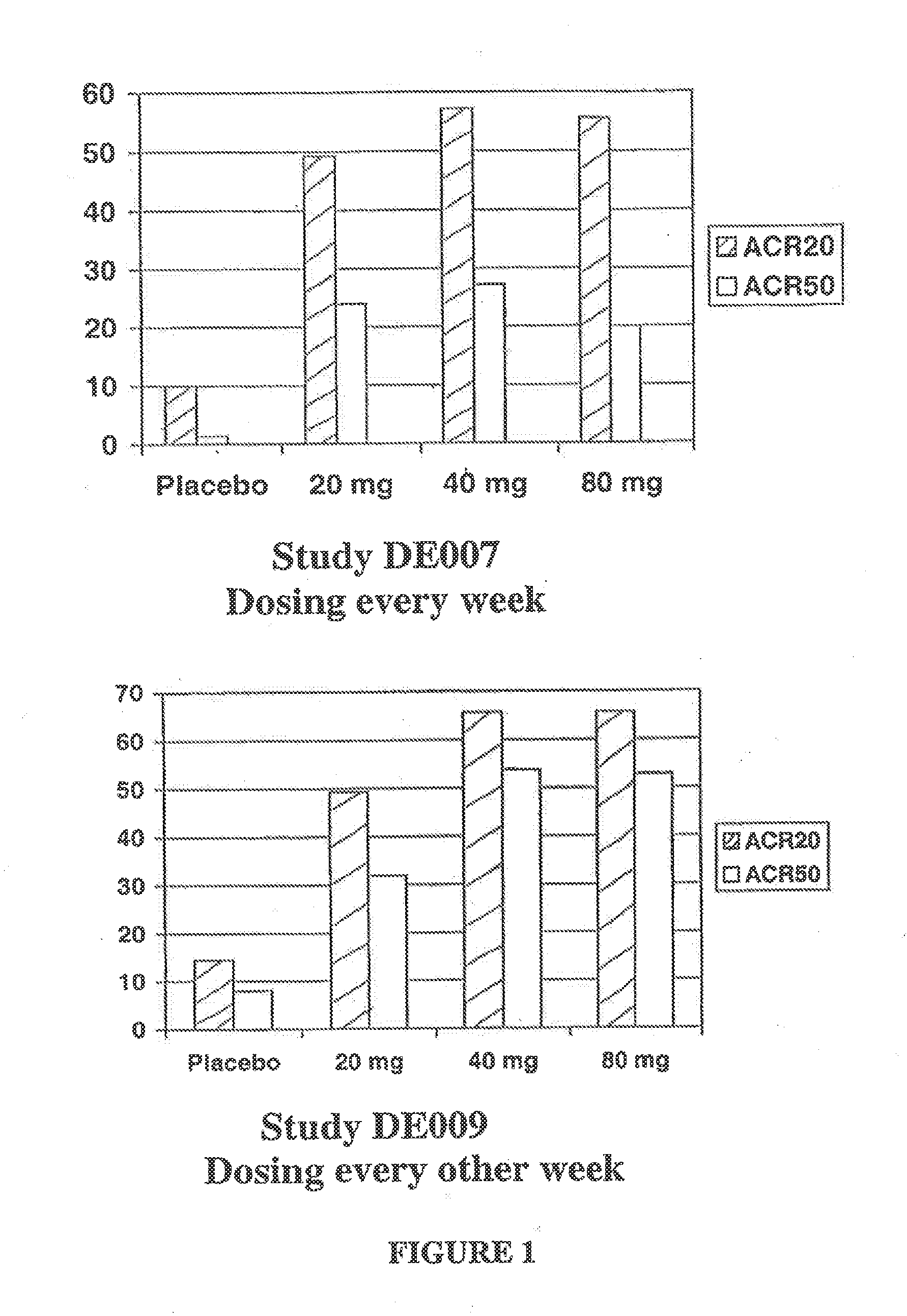
[0127]This study enrolled two hundred eighty-four patients with RA and was designed to determine the optimal total body dose of subcutaneously adminstered D2E7. Patients were randomized to receive either 20, 40, or 80 mg D2E7 or placebo weekly for twelve weeks, after which time placebo-treated patients were switched blindly to 40 mg D2E7 / week.
[0128]Approximately 49% of patients reached ACR20 at 20 mg, 55% of patients reached ACR20 at 40 mg, and 54% of patients reached ACR20 at 30 mg, while only 10% of patients receiving placebo reached ACR20 (set forth in FIG. 1A). Approximately 23% of patients reached ACR50 at 20 mg, 27% of patients reached ACR50 at 40 mg, and 20% of patients reached ACR50 at 80 mg, and only 2% of patients receiving placebo reached ACR50. These data illustrate that subcutaneous D2E7, particularly at a dose of 40 mg / week, generates a good response.
example 3
Biweekly, Subcutaneous Administration of an Anti-TNFα Antibody Biweekly, Subcutaneous Administration of B2E7
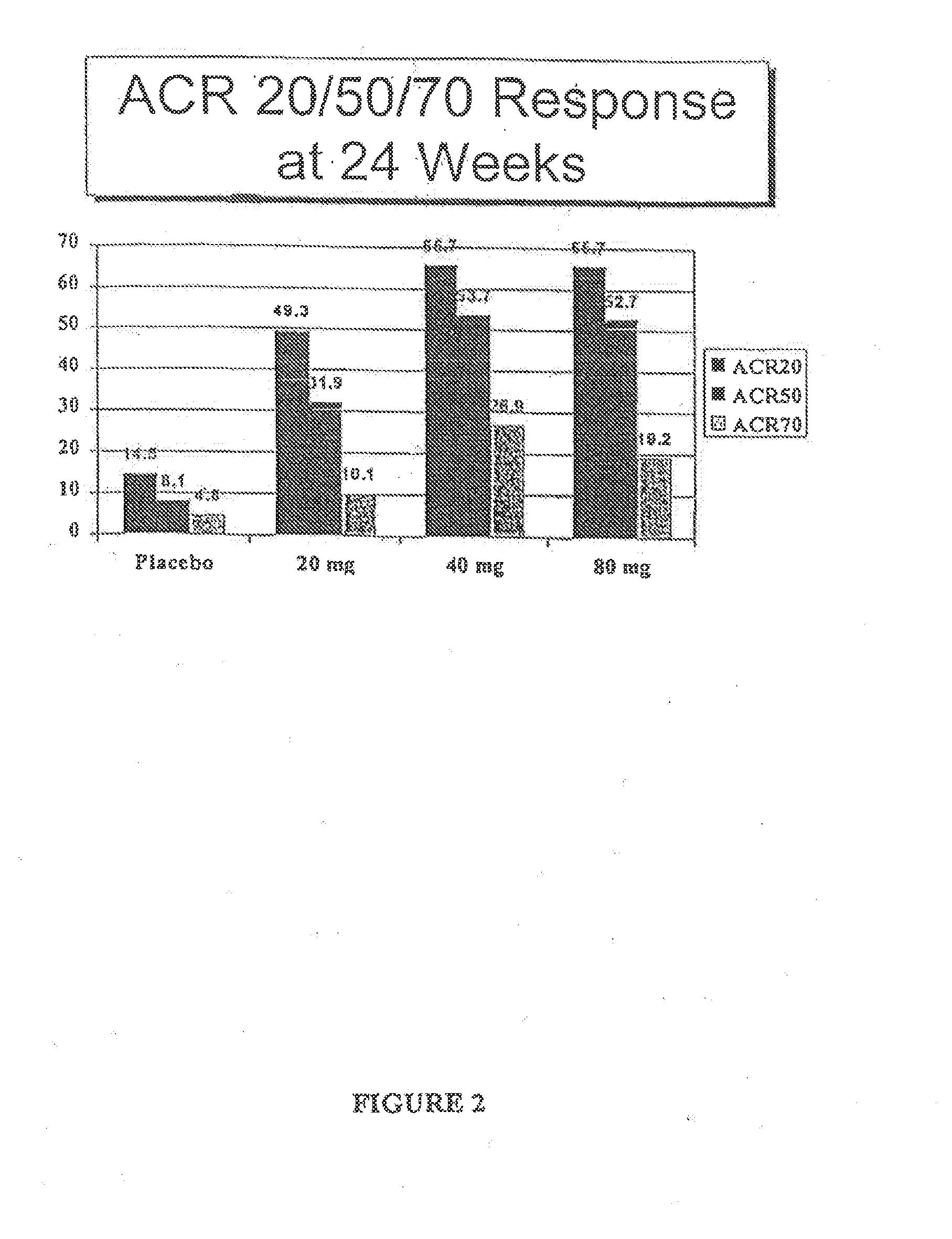
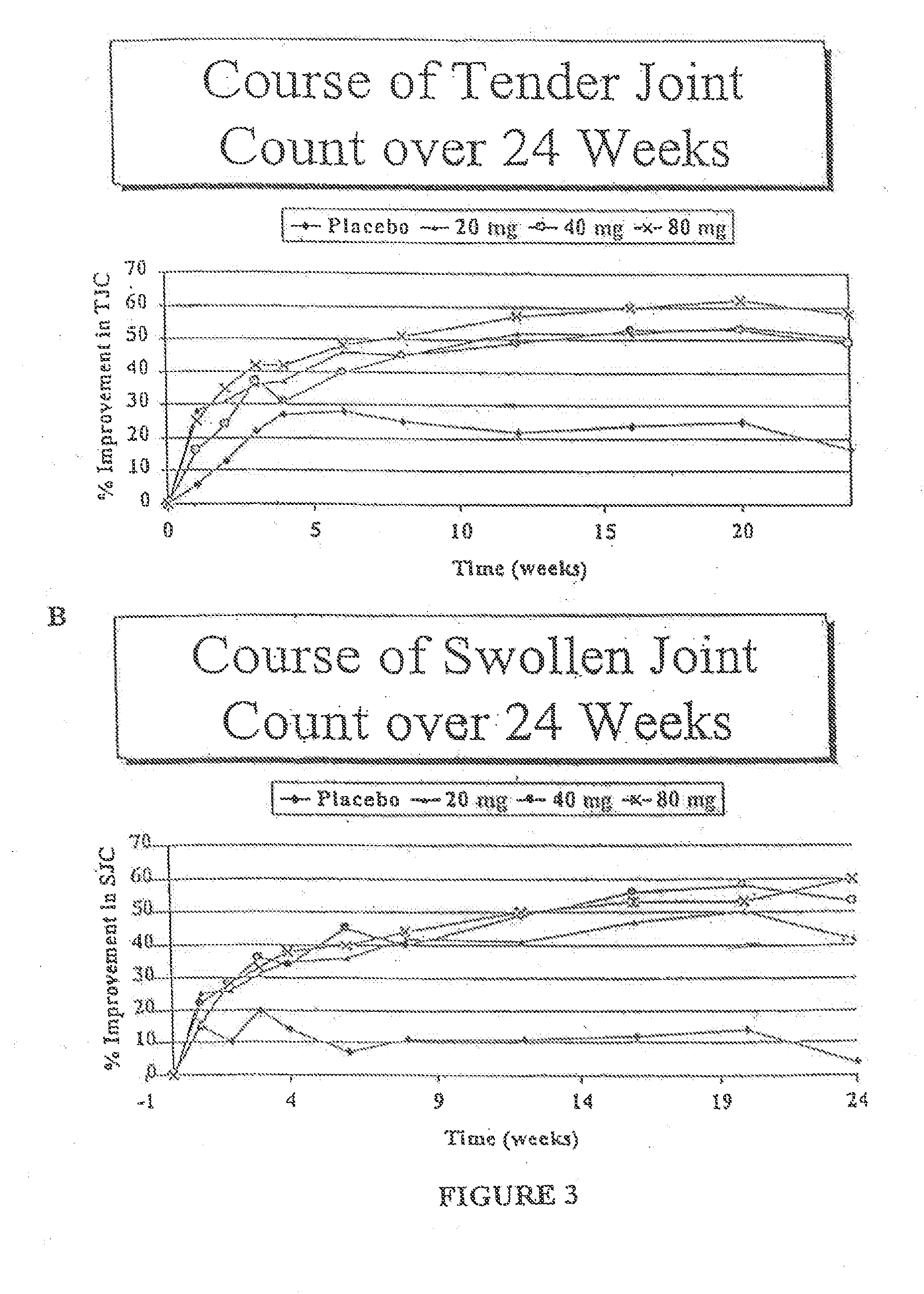
[0129]The clinical effects, safety, immunogenicity, and tolerance of RA patients with partial responses to MTX following every other week subcutaneous (s.c.) injections of placebo or D2E7 at several dose levels for up to twenty-four weeks in conjunction with continued MTX treatment was investigated.
Study Design
[0130]A placebo-controlled, double-blind, randomized, multi-center study in patients with RA, who had insufficient efficacy or tolerability to MTX was performed. During the course of the trial, patients were continued on a stable dose of MTX with dose ranges specified in the inclusion criteria described below.
[0131]This study consisted of two portions: 1) a “wash-out period” of four weeks prior to the administration of the first dose medication, during which time DMARDs (except for MTX) were withdrawn; and 2) a “placebo controlled period” during which time patients were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com