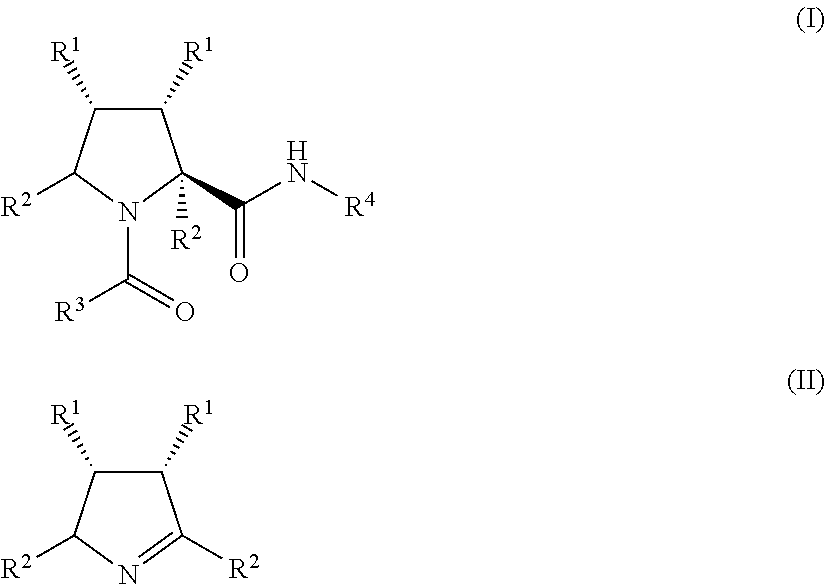
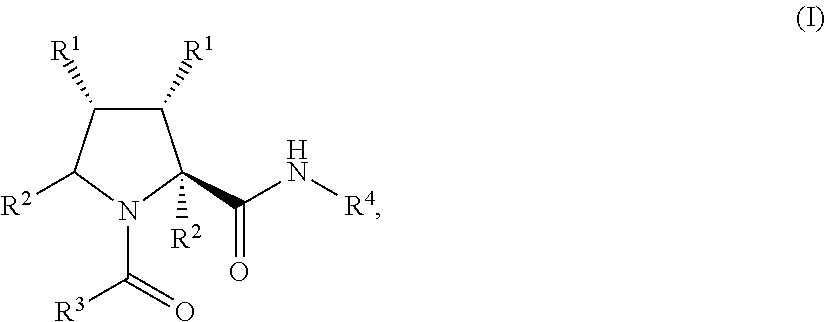
Process for the preparation of substituted prolyl peptides and similar peptidomimetics
a technology of substituted prolyl peptides and peptidomimetics, which is applied in the direction of tetrapeptide ingredients, tripeptide ingredients, biocides, etc., can solve the problems of poor and/or unpredictable diastereoselectivity of known multicomponent reactions for the preparation of proline derivative comprising peptides and peptidomimetics, and achieves excellent yield and enantiomeric excess, high efficiency, and effectiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0118]
Compound 5a:
[0119]General procedure 2 was followed using 3-azabicyclo[3,3,0]oct-2-ene ((3S,7R)-4, 76 mg, 0.70 mmol), acetic acid (55 mg, 52 μl, 0.91 mmol) and t-butyl isocyanide (76 mg, 103 μl, 0.91 mmol) giving 5a as a white solid, yield 73%.
[0120]93:7 d.r. [HP-1, t (major)=14.852 min, t (minor)=16.773 min]; 95% ee [CP Chirasil-DEX CB, t (minor)=20.449 min, t (major)=20.860 min]; [α]D20=−47.8° (c=0.34, MeCN). 1H NMR (400.1 MHz, CDCl3): δ 6.58 (bs, 1H), 4.28 (d, J=2.1 Hz, 1H), 3.70 (dd, J=8.3, 10.6 Hz, 1H), 3.24 (dd, J=4.5, 10.6 Hz, 1H), 2.96-2.93 (m, 1H), 2.91-2.82 (m, 1H), 2.01 (s, 3H), 1.93-1.78 (m, 2H), 1.71-1.42 (m, 2H), 1.41-1.31 (m, 2H), 1.25 (s, 9H); 13C NMR (100.6 MHz, CDCl3) δ 170.5, 170.0, 66.8, 54.4, 51.0, 45.0, 42.7, 32.5, 32.3, 28.7, 25.7, 22.6; IR (neat): νmax (cm−1)=3277 (m), 2957 (m), 1668 (s), 1630 (s), 1549 (s), 1447 (s), 1420 (s), 1223 (s), 667 (m), 606 (m); HRMS (ESI+) calcd for C14H24N2O2 ([M+H]+) 253.1916. found 253.1925.
example 2
[0121]
Compound 5b:
[0122]General procedure 2 was followed using 3-azabicyclo[3,3,0]oct-2-ene ((3S,7R)-4, 76 mg, 0.70 mmol), benzoic acid (111 mg, 0.91 mmol) and t-butyl isocyanide (76 mg, 103 μl, 0.91 mmol) giving 5b as a white solid, yield 73%.
[0123]93:7 d.r. [HP-1, t (major)=23.672 min, t (minor)=25.601 min]; 95% ee [Daicel Chiralpak AD-H, hexane / 2-propanol=96 / 4, v=1.0 mL / min1, λ=254 nm, t (minor)=10.698 min, t (major)=11.620 min]; [α]D20=−53.7° (c=0.34, MeCN). 1H NMR (400.1 MHz, CDCl3): δ 7.49-7.38 (m, 5H), 6.66 (bs, 1H), 4.54 (d, J=2.8 Hz), 3.72 (dd, J=11.4, 7.8 Hz, 1H), 3.23 (d, J=11.0, 1H), 3.15-3.10 (m, 1H), 2.73-2.58 (m, 1H), 1.96-1.82 (m, 1H), 1.82-1.69 (m, 1H), 1.68-1.41 (m, 3H), 3.15-3.10 (m, 1H), 1.28 (s, 9H), 1.24-1.06 (m, 1H); 13C NMR (100.6 MHz, CDCl3) δ170.3, 170.1, 136.3, 130.1, 128.4, 126.9, 67.1, 60.4, 55.9, 51.1, 44.2, 43.3, 33.0, 32.7, 28.7, 26.2; IR (neat): νmax (cm−1)=3310 (m), 2961 (m), 1674 (s), 1618 (s), 1416 (s), 1223 (s), 698 (s); HRMS (ESI+) calcd for C19...
example 3
[0124]
Compound 5c:
[0125]General procedure 2 was followed using 3-azabicyclo[3,3,0]oct-2-ene ((3S,7R)-4, 76 mg, 0.70 mmol), 3-furoic acid (102 mg, 0.91 mmol) and isopropyl isocyanide (63 mg, 86 μl, 0.91 mmol) giving 5c as a white solid, yield 75%.
[0126]92:8 d.r. [HP-1, t (major)=21.290 min, t (minor)=23.012 min] 94% ee [Daicel Chiralpak AD-H, hexane / 2-propanol=90 / 10, v=1.0 mL·min1, λ=254 nm, t (minor)=7.417 min, t (major)=12.039 min]; [α]D20=−33.3° (c=0.30, MeCN). 1H NMR (400.1 MHz, CDCl3): δ 7.80 (bs, 1H), 7.43 (bs, 1H), 6.72 (bs, 1H), 6.51 (d, J=6.3 Hz, 1H), 4.56 (d, J=2.3 Hz, 1H), 4.03 (oct, J=7.1 1H), 3.88 (dd, J=10.4, 8.3 Hz, 1H), 3.53 (dd, J=10.4, 3.8 Hz, 1H), 3.09-3.01 (m, 1H), 2.95-2.84 (m, 1H), 2.00-1.84 (m, 2H), 1.74-1.65 (m, 1H), 1.64-1.54 (m, 1H), 1.53-1.43 (m, 1H), 1.43-1.33 (m, 1H), 1.17 (d, J=6.3 Hz, 3H) 1.13 (d, J=6.3 Hz, 3H); 13C NMR (100.6 MHz, CDCl3) δ 170.1, 163.2, 144.3, 142.8, 121.8, 110.4, 66.8, 54.8, 44.4, 43.3, 41.3, 32.4, 32.2, 25.6, 22.5, 22.4; IR (neat): ν...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Structure | aaaaa | aaaaa |
| Selectivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com