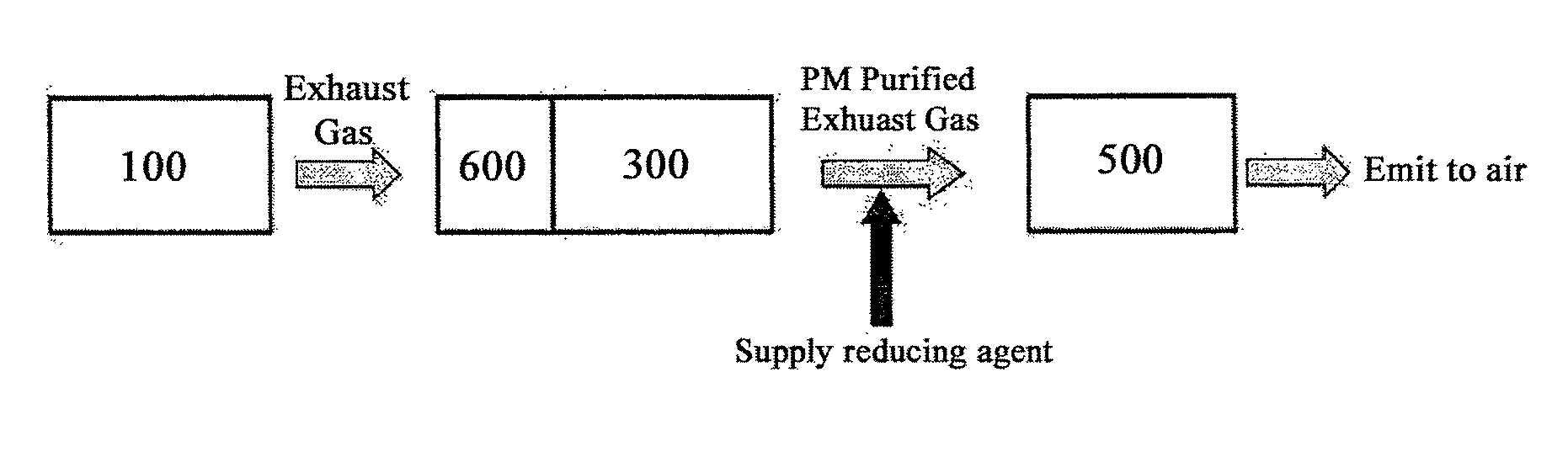
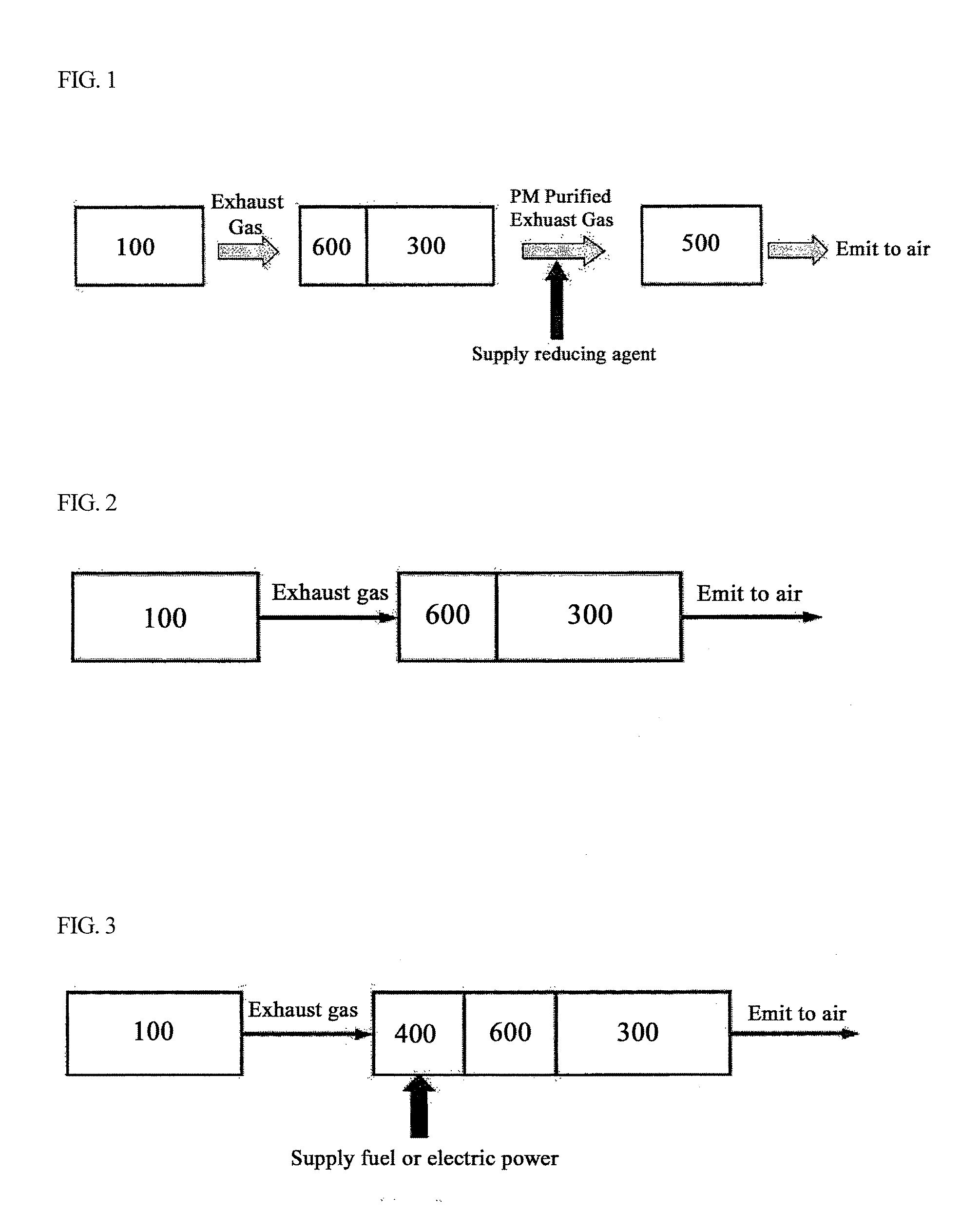
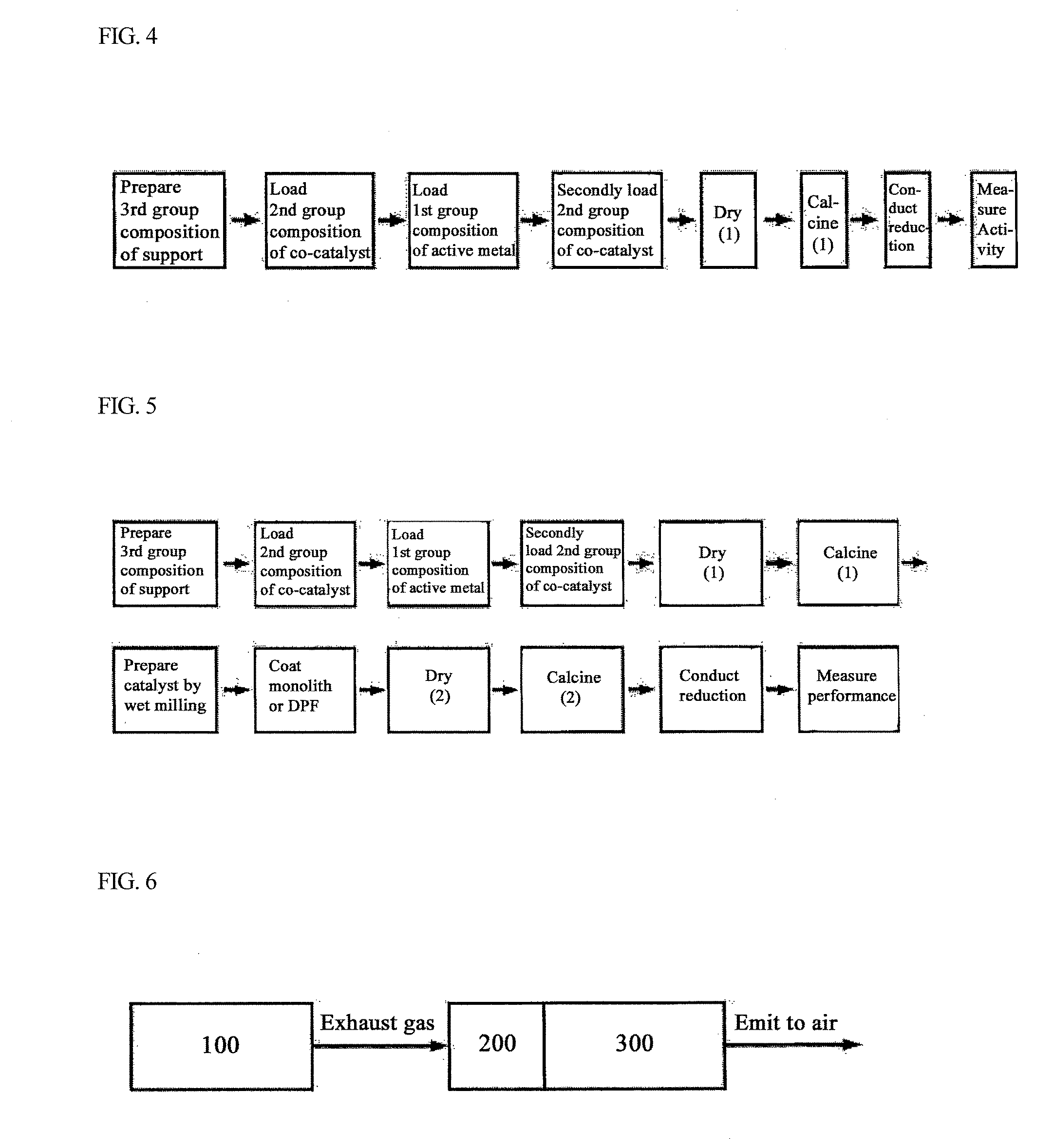
Bifunctional Catalyst for Decomposition and Oxidation of Nitrogen Monoxide, Composite Catalyst Including the Same for Apparatus to Decrease Exhaust Gas, and Method for Preparation Thereof
a nitrogen monoxide and bifunctional technology, applied in the direction of physical/chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, separation processes, etc., can solve the problems of catalyst activity being too low to be used in a catalyst system, catalyst durability is insufficient, and maintenance costs increas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0095]A catalyst was prepared by the same procedure described in Example 1, except that ZrO2 was used as a support of the catalyst (referred to as KOC-2).
[0096]For KOC-2 catalyst prepared as described above, after conducting reduction at 300° C. for 30 minutes using a reductant gas (10 vol %, H2 / N2) and before conducting NOx decomposition experiments, performance of the catalyst was evaluated. FIG. 9 illustrates NOx decomposition efficiency while FIG. 10 shows NO2 generation efficiency.
[0097]As a result of determining catalyst activity, it can be seen that NOx decomposition capability was greatly improved as compared to Pt[5] / γ—Al2O3, and NOx decomposition capability and NO2 generation selectivity were greatly improved as compared to commercially available catalysts.
example 3
[0098]Pt[2]-W[5] / TiO2 was prepared by loading, drying and calcining active metal and co-catalyst according to the same procedures described in Example 1. In order to improve NOx decomposition capability and durability, tungsten (W) among a second group of co-catalysts was additionally loaded in an amount of 1.0 wt. % relative to a total weight of the support. Then, drying, calcining and reduction were conducted to prepare a catalyst. Such prepared catalyst was indicated to as KOC-3.
[0099]For KOC-3 catalyst prepared as described above, after conducting reduction at 300° C. for 30 minutes using a reductant gas (10 vol %, H2 / N2) and before conducting NOx decomposition experiments, activity of the catalyst was evaluated. FIG. 9 illustrates NOx decomposition efficiency while FIG. 10 shows NO2 generation efficiency.
[0100]As a result of determining catalyst activity, it can be seen that NOx decomposition capability and NO2 generation selectivity were greatly improved as compared to Pt[5] / γ...
example 4
[0101]A slurry solution was prepared by wet milling the catalyst KOC-1 powder according to Example 1. Ceramic monolith (400 cpi) was immersed into the slurry solution to coat a surface of the monolith with catalyst component. Immersion and drying were repeated until an amount of the catalyst coating reached 60 g / L. After drying, the coated monolith was subjected to calcination at 550° C. for 4 hours in an air atmosphere, then, reduction at 300° C. for 1 hour in a 10 vol % hydrogen / nitrogen atmosphere, thereby forming a DOC.
[0102]By combining the completed DOC (diameter of 14 cm, length of 7.3 cm, 400 cpi) with ceramic DPF (diameter of 14 cm, length of 23 cm, 200 cpi), an integrated can was fabricated and used to manufacture a contaminant reducing device.
[0103]The exhaust gas reducing device was mounted on an automobile, for example, commercially available under the trade mane CARNIVAL (with TCI engine, KIA Motors, Korea) (see FIG. 11) and PM trapping amount depending upon time was m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com