Nanoporous Silica Polyamine Composites with Surface-Bound Zirconium (IV) and Methods of Use
a technology of nanoporous silica and composites, which is applied in the direction of water/sewage treatment by ion exchange, ion exchangers, chemistry apparatus and processes, etc., can solve the problems of large amount of sludge for ultimate disposal, arsenic cannot be recovered, and the arsenic removal process is difficult, etc., to achieve the effect of reducing selectivity and reducing uptake performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials
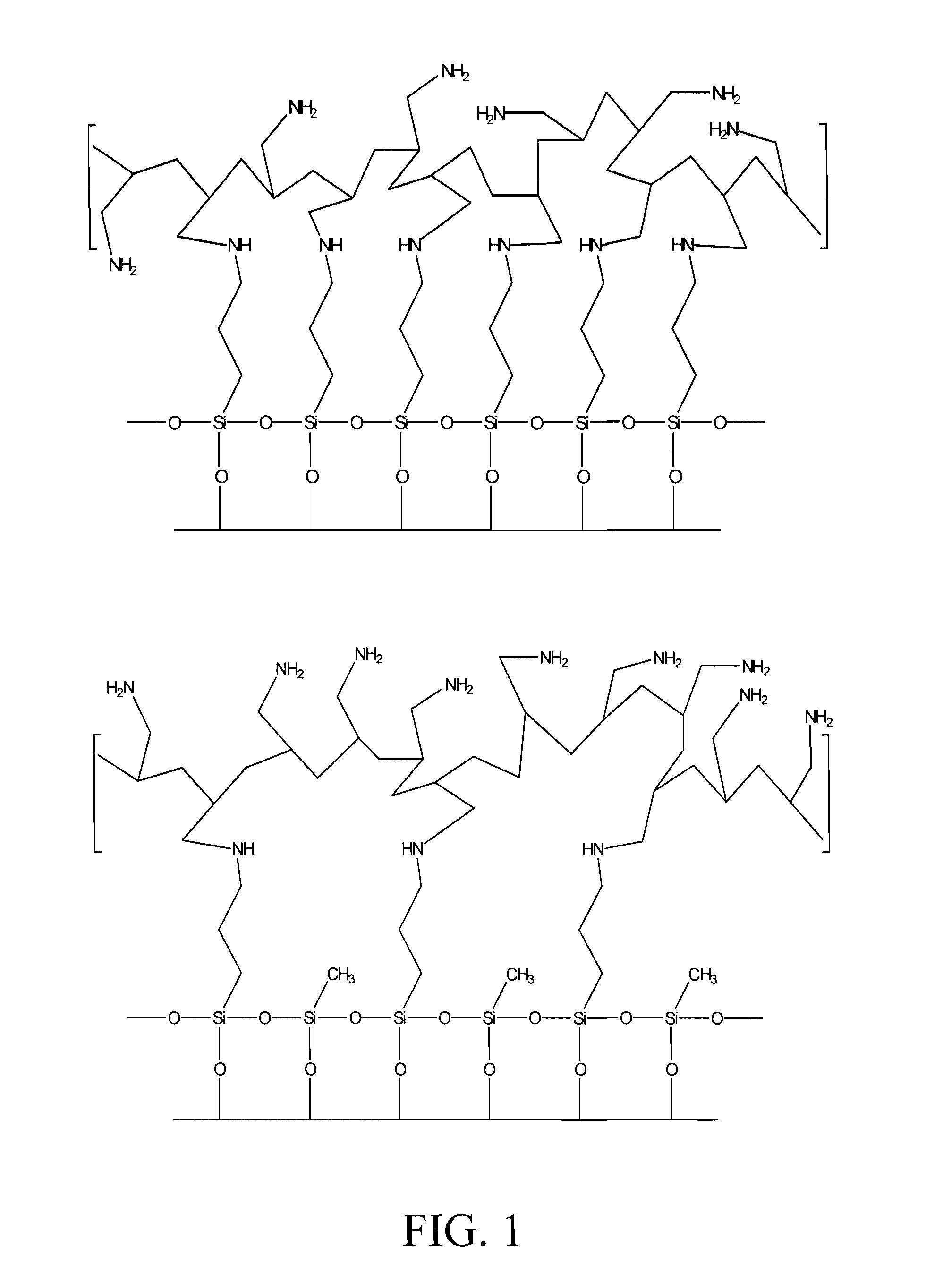
[0036]Silica gel (267 A° pore diameter, 2.82 mL / g pore volume, 84.7% porosity, 422 m2 / g surface area) was obtained from INEOS enterprises Ltd., UK, and was sieved to 150-250 microns. All chemicals were reagent grade and were purchased from Sigma-Aldrich Co. Stock solutions of Zr(IV), Fe(III), Th(IV), As(III), As(V), Se(IV), and Se(VI) were prepared using zirconyl chloride (ZrOCl2.8H2O), ferric sulfate (Fe2(SO4)3.4H2O), thorium nitrate (Th(NO3)4.xH2O), sodium meta-arsenite (NaAsO2), sodium hydrogen arsenate (Na2HAsO4.7H2O), sodium selenite (Na2SeO3), and sodium selenate (Na2SeO4) respectively. Solution pH was adjusted from the intrinsic pH, where necessary, using hydrochloric acid and sodium hydroxide. Stripping and recovery of arsenic was achieved with 2M—H2SO4. Metal standards for AA and ICP analysis were obtained from Fisher Scientific Co.
[0037]Infrared characterization of modified composites was carried out with a Thermo-nicolet Nexus 670 FT-IR spectrophotometer as KBr p...
example 2
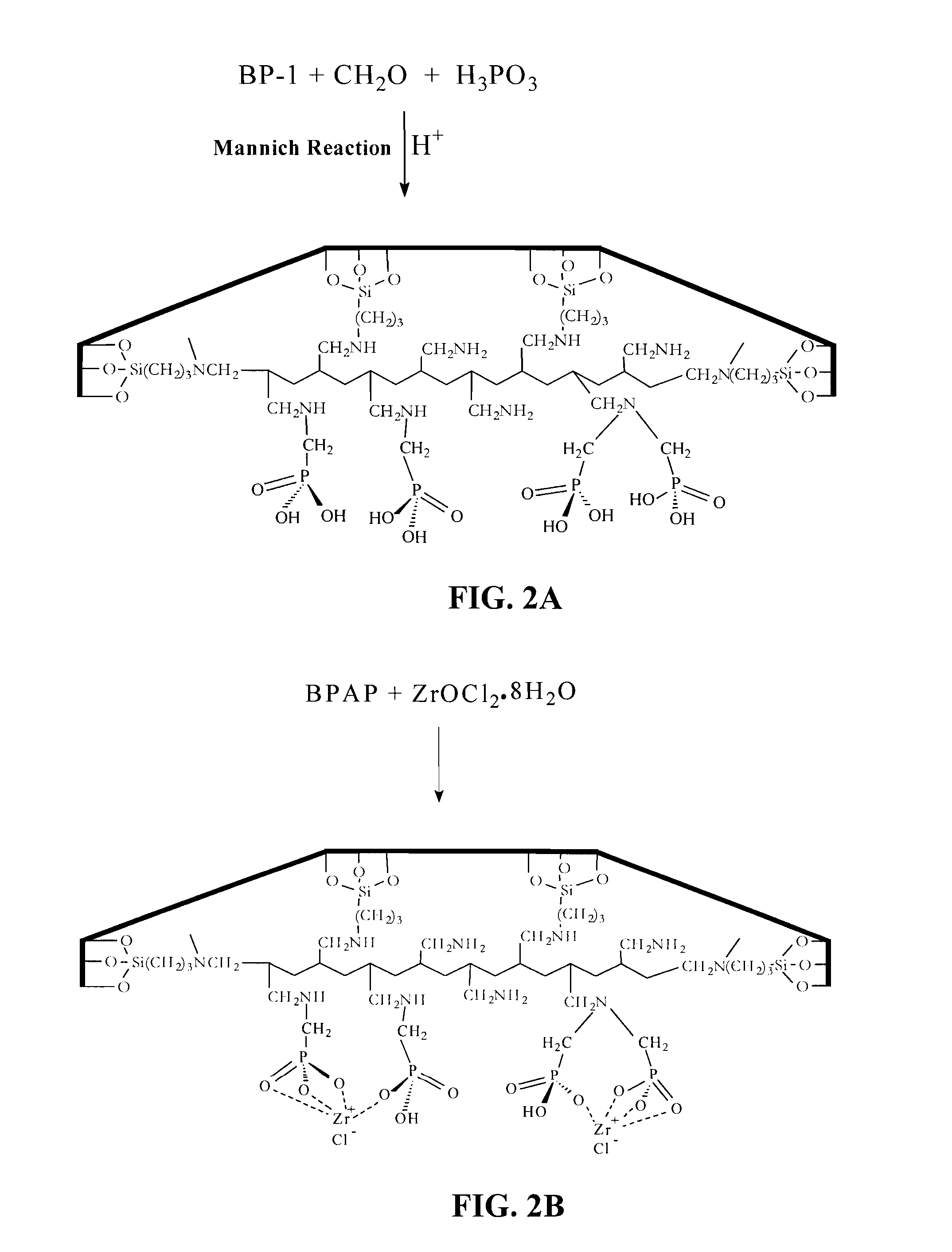
Synthesis of BPAP and BPAPM (FIG. 2a) by the Mannich Reaction (Moedritzer, 1966)
[0040]10 g of BP-1 or BP-1M was mixed with a reagent solution of 30 mL-2N—HCl and 10 g of phosphorus acid (H3PO3) in a 250 mL flask equipped with an overhead stirrer. The flask was heated to 95° C., and 9 mL of formaldehyde (CH2O) solution (37.7%) was gradually added with stirring. The reaction mixture was heated at 95° C. for 24 h. The flask was cooled and the product was filtered. The resulting composite was washed three times with 40 mL of water, once with 40 mL of 1M—NaOH, three times with 40 mL of water, once with 40 mL of 1N—H2SO4, two more times with 40 mL of water, twice with 40 mL of methanol and dried to a constant mass at 65° C. A mass gain of 20 and 22% was obtained starting with BP-1 and BP-1M respectively.
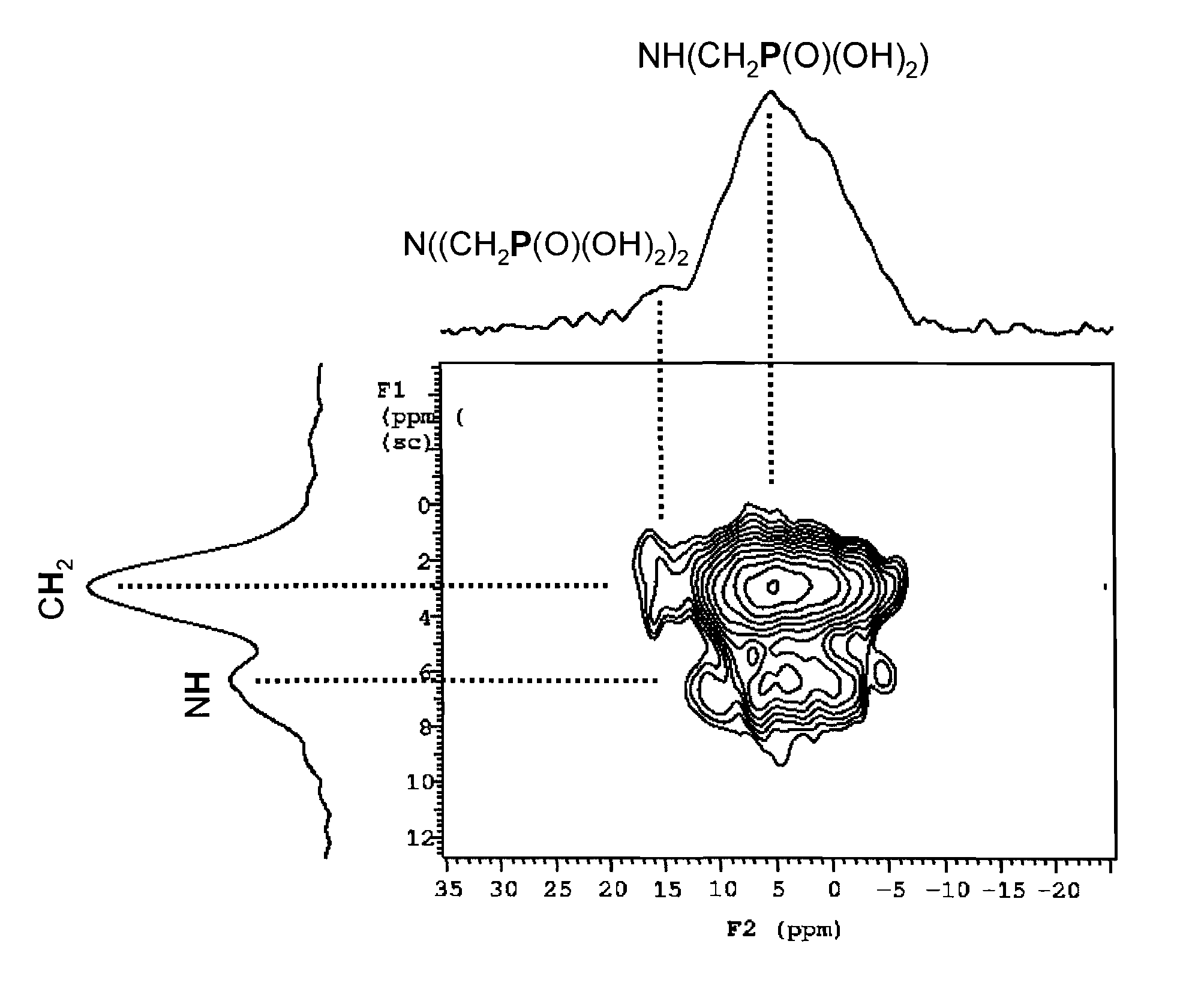
[0041]Elemental Analysis: BPAP made from BP-1: 14.20% C, 3.03% H, 3.36% N, 4.34% P
[0042]BPAP made from BP-1M: 12.08% C, 2.99% H, 2.21% N, 5.56% P
IR spectra (KBr pellet): 2650(m)cm−1(νP—OH)...
example 3
Synthesis of ZrBPAP (FIG. 2b)
[0043]10 g of the BPAP composite was mixed with a reagent solution containing 40 mL of 1N—HCl and 4.24 g of ZrOCl2.8H2O. The reaction mixture was stirred at room temperature for 24 h. After 24 h the product was filtered. The resulting composite was washed two times with 50 mL of water, once with 2N—HCl, two more times with 50 mL water, twice with 50 mL methanol and dried to a constant mass at 65° C. The zirconium loading was determined by analyzing the zirconium feed solution and the final zirconium solutions after all the washes were performed. The analysis of zirconium was done via an ICP / AES method. The zirconium loading on BPAP was found to be 1.12 mmol / g which fits well with a mass gain of 17%. Although, zirconyl chloride forms a tetramer [Zr4(OH)8(H2O)16]8+ in solution, the immobilized zirconium (IV) is thought to bind to the oxygen atom(s) of two phosphonic acid moieties. This is supported by the data obtained from the elemental analysis, ICP / AES,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com