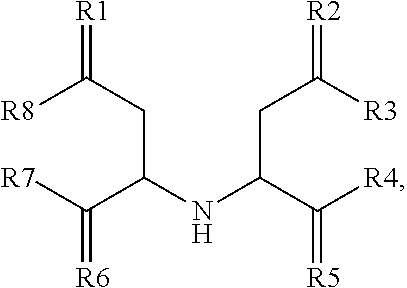
Electrolyte composition
a technology of electrolyte composition and composition, which is applied in the direction of coatings, surface reaction electrolytic coatings, semiconductor devices, etc., can solve the problems of high effort and effort to replace the toxic complexing agent cyanide, and achieve the effect of high deposition rate and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034]The following specific embodiments of the present invention do not limit the scope thereof, but are for illustration.
Comparative Composition 1
[0035]An electrolyte composition composed of the following components was tested:
Silver as silver methane sulfonate30 g / LTripotassium citrate monohydrate40 g / LPotassium hydroxide65 g / L5,5-Dimethyl hydantoin130 g / L
[0036]The pH of the composition was 10.3.
Exemplary Composition 1
[0037]An electrolyte composition composed of the following components was tested:
Silver as silver methane sulfonate30 g / LTripotassium citrate monohydrate40 g / LPotassium hydroxide65 g / L5,5-Dimethyl hydantoin130 g / L Tetrasodium iminodisuccinate10 g / L
[0038]The pH of the composition was 10.3.
Test Procedure:
[0039]The above-mentioned electrolyte according to example 1 of the invention and the electrolyte according to the comparative example were filled into separate beakers, and in each beaker a brass sheet having the dimensions of 10 cm×7 cm was electroplated on one sid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com