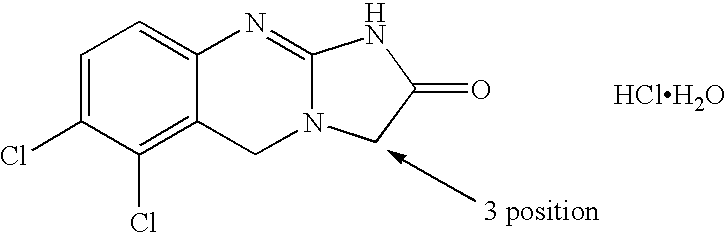
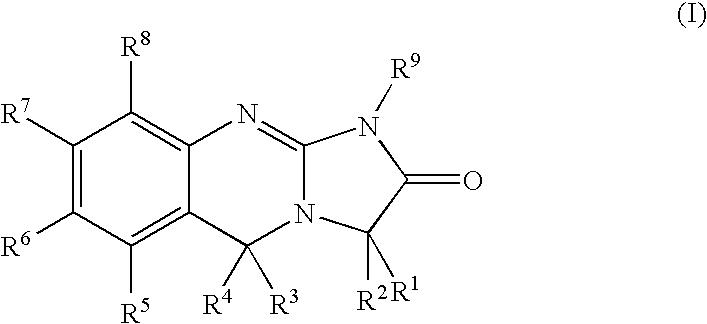
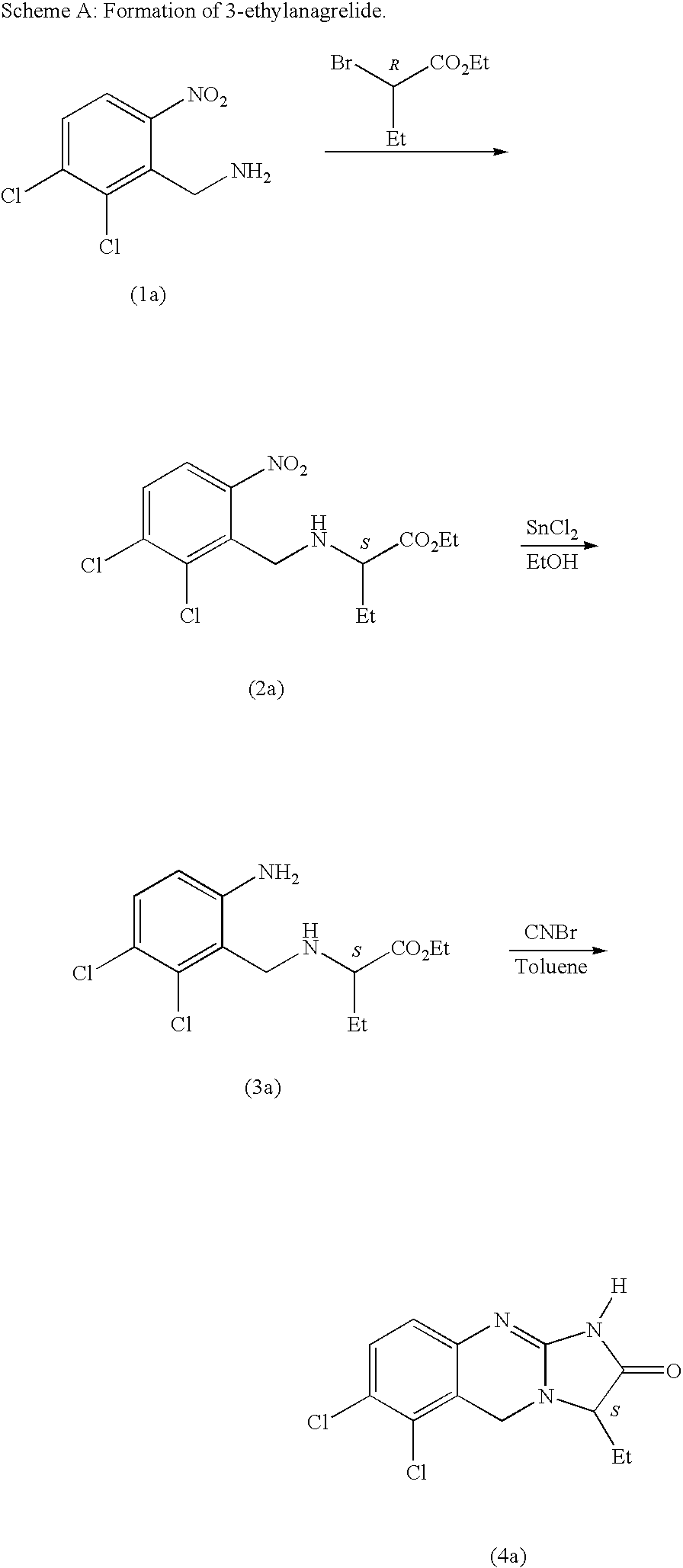
Substituted quinazolines
a technology of quinazoline and quinazoline, which is applied in the field of substituted quinazoline, can solve the problems of limiting its utility, significant proportion of patients, and 50% of patients who cannot tolerate the drug during long-term treatment, and achieves the effect of reducing cardiac toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparative IC50 Data on Anagrelide and Some 3-Alkyl Substituted Analogues as PDE III Inhibitors and Anti-Megakaryocytic Agents
[0109]The table below shows the comparative activity of anagrelide and its analogues with respect to their effects on megakaryocytopoeisis (the process giving rise to blood platelets) and PDE III (inhibition of which leads to adverse cardiovascular responses).
Comparative In Vitro Assessment of Potential Therapeutic and Adverse Effects of Anagrelide and its Analogues
[0110]
IC50 for anti-IC50 for PDE IIImegakaryocyticinhibitionBenefit ratio(platelet lowering)(cardiovascular(therapeutic toCompoundactivityeffects)adverse effects)Anagrelide 27 nM 32 nM*0.024:1 3-hydroxy 44 nM 0.7 nM0.016:1anagrelide3,3 dimethyl164 nM166 nM 1:1anagrelide3-spirocyclo547 nM797 nM 1.45:1propylanagrelide*Pharmacokinetically adjusted value based on three-fold greater systemic exposure to active metabolite (3-hydroxy anagrelide) than to the drug itself in man.
[0111]Assessment of the in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| chemical structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com