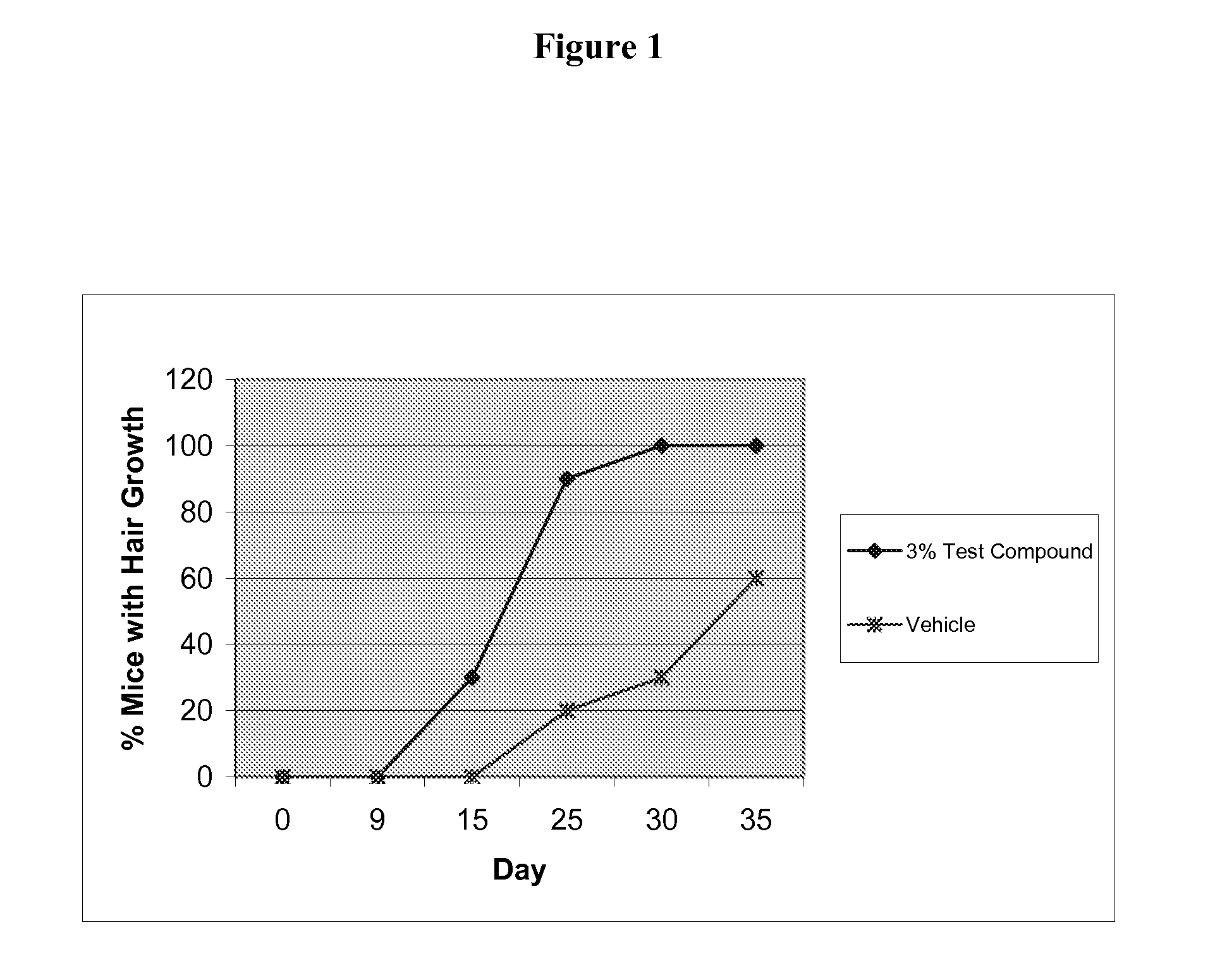
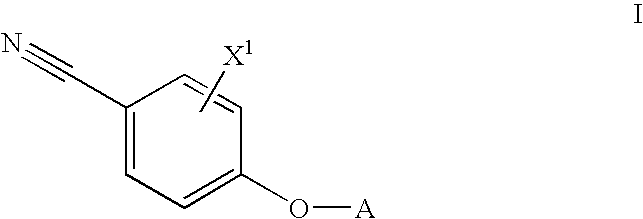
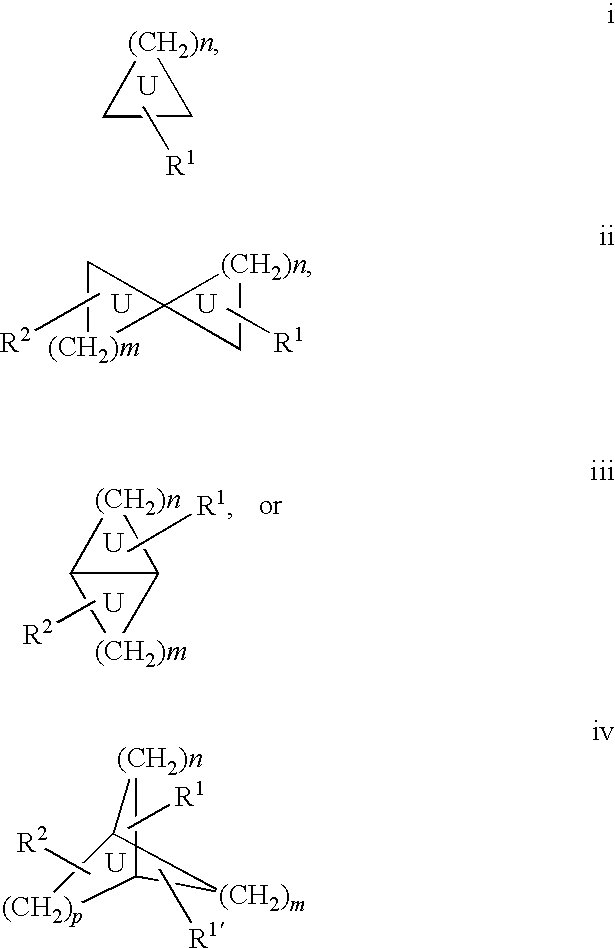
Androgen modulators
a technology of androgen receptors and modulators, which is applied in the field of androgen receptor modulators, can solve the problems of balding, common problem that medical science has yet to alleviate, and gradual changes in the width and length of the hair shaft, so as to enhance the lean meat to fat ratio in the animal, improve the effect of feeding efficiency and increase the growth ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
4-(5-Hydroxy-5-methyl-bicyclo[2.2.1]hept-2-yloxy)-2-trifluoromethyl-benzonitrile
[0197]
[0198]NaH (0.10 g, 1.75 mmol, 60% in mineral oil) was suspended in 15 ml of dry THF (tetrahydrofuran) at 0° C. under nitrogen (N2) gas, then 4-fluoro-2-(trifluoromethyl)-benzonitrile (0.3 g, 1.59 mmol) was added, this mixture was stirred at 0° C. under N2 for 10 minutes, before adding 2-methyl-bicyclo[2.2.1]heptane-2,5-diol (0.23 g, 1.59 mmol). The reaction mixture was stirred at 0° C. for 2 hours (h), then room temperature (RT) for 1 h. It was quenched with 50 ml of distilled water, extracted with ethyl acetate (3×30 ml), the organic layer was washed with saturated NaHCO3 (three times), the solvent was removed to yield the crude product, it was purified by column with hexane:ethyl acetate=1:1 as the elute.
[0199](MS: 312.1 (M+1 for C16H16NF3O2) LCMS: C-18 Column (25% H2O / 75% CH3CN), Ret. Time: 1.31 min Purity: 100%)
example 2
4-(trans-2-Methyl-cyclopentyloxy)-2-trifluoromethyl-benzonitrile
[0200]
[0201]Trans-2-Methylcylopentanol (50 mg, 0.5 mmol) was added to a flame dried round bottom flask under nitrogen containing 0.5 mL of dry tetrahydrofuran (“THF”) and cooled to 0° C. A 1.0 M solution of potassium t-butoxide in t-butanol (0.5 mL) was added dropwise and the reaction mixture stirred at 0° C. for 0.5 h. The above solution was then transferred via syringe to a flask containing 2-Trifluoromethyl-4-fluoro-benzonitrile (95 mg, 0.5 mmol) in 0.5 mL of THF at 0° C. The mixture was stirred at 0° C. for 3 h, warmed to room temperature and stirred for 16 h. The reaction mixture was then cooled to 0° C., poured into a seperatory funnel containing 8 mL of water, and extracted with 10 mL of methyl tert-butyl ether. The organic phase was washed with water, brine, dried (MgSO4), concentrated in vacuo, and the residue purified by reverse phase HPLC (Shimadzu) to give 112 mg 4-(trans-2-Methyl-cyclopentyloxy)-2-trifluoro...
example 3
4-(2-(R)-Phenyl-1-(S)-cyclohexyloxy)-2-trifluoromethyl-benzonitrile
[0202]
[0203]The title compound was prepared according to the procedure described in Example 2, except that 1-(S)-2-(R)-Phenyl-cyclohexyanol was used as the starting alcohol to give 66 mg of the desired product. GC / MS: 345 (M / Z for C20H18F3NO).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com