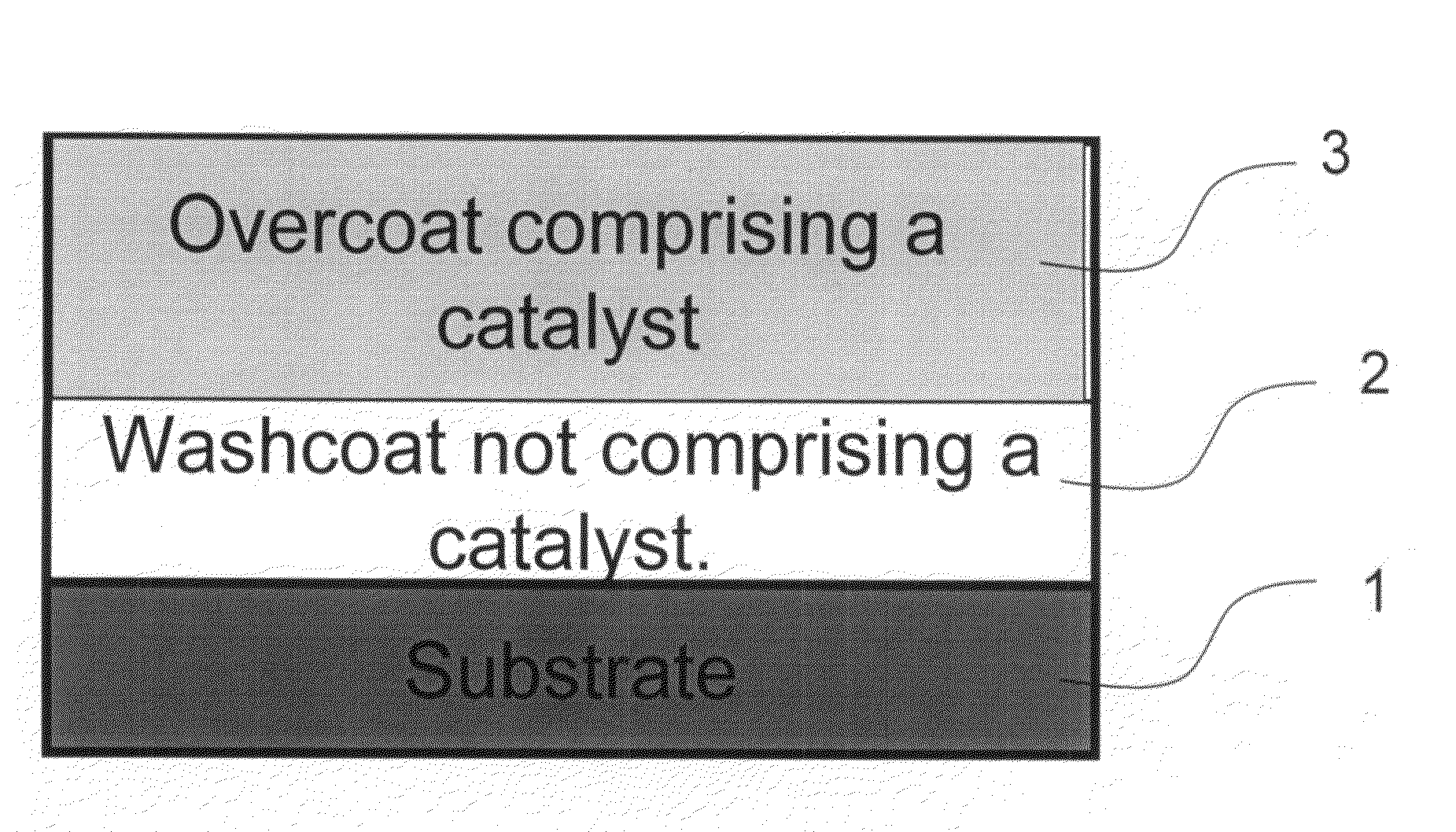
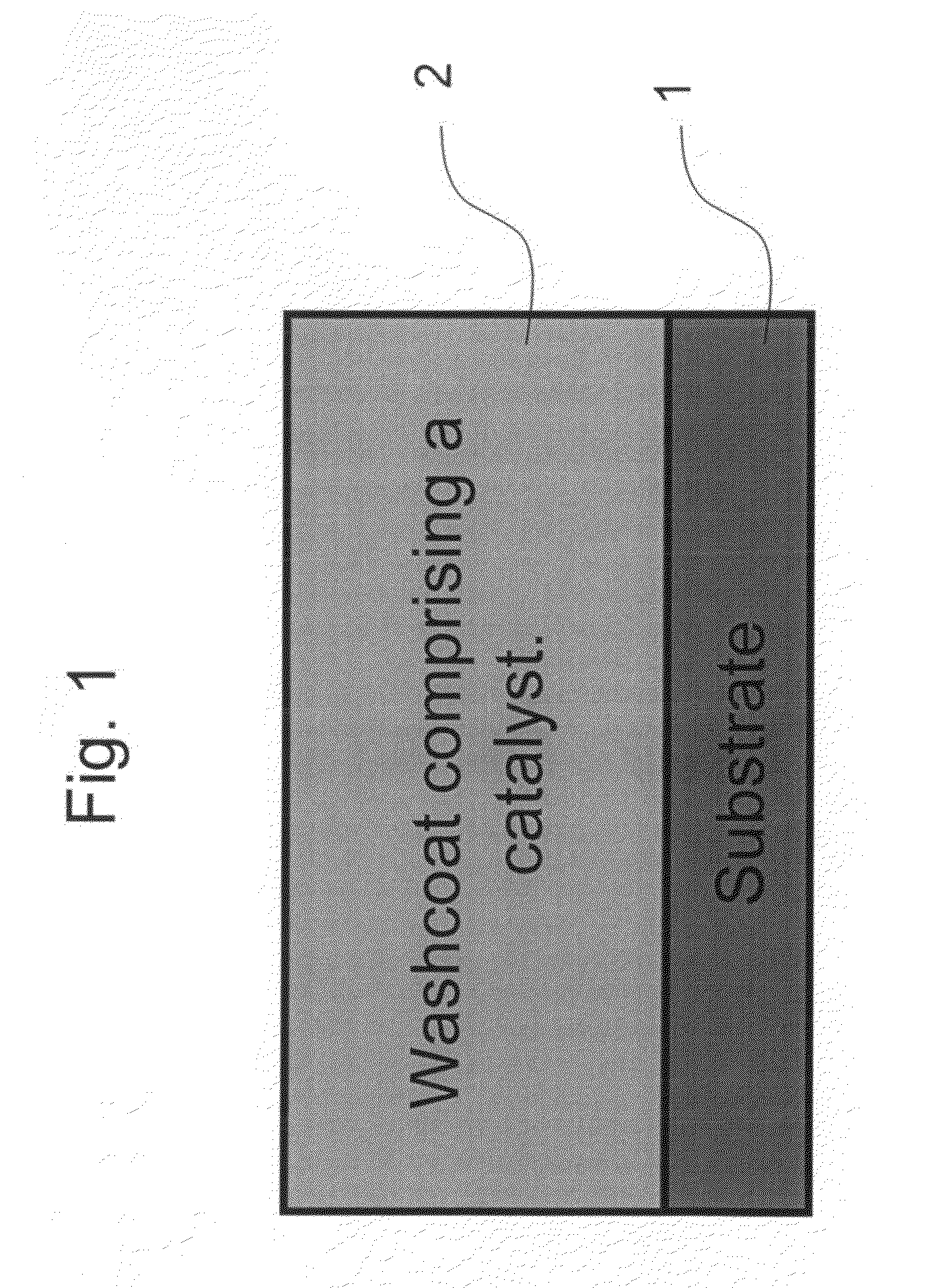
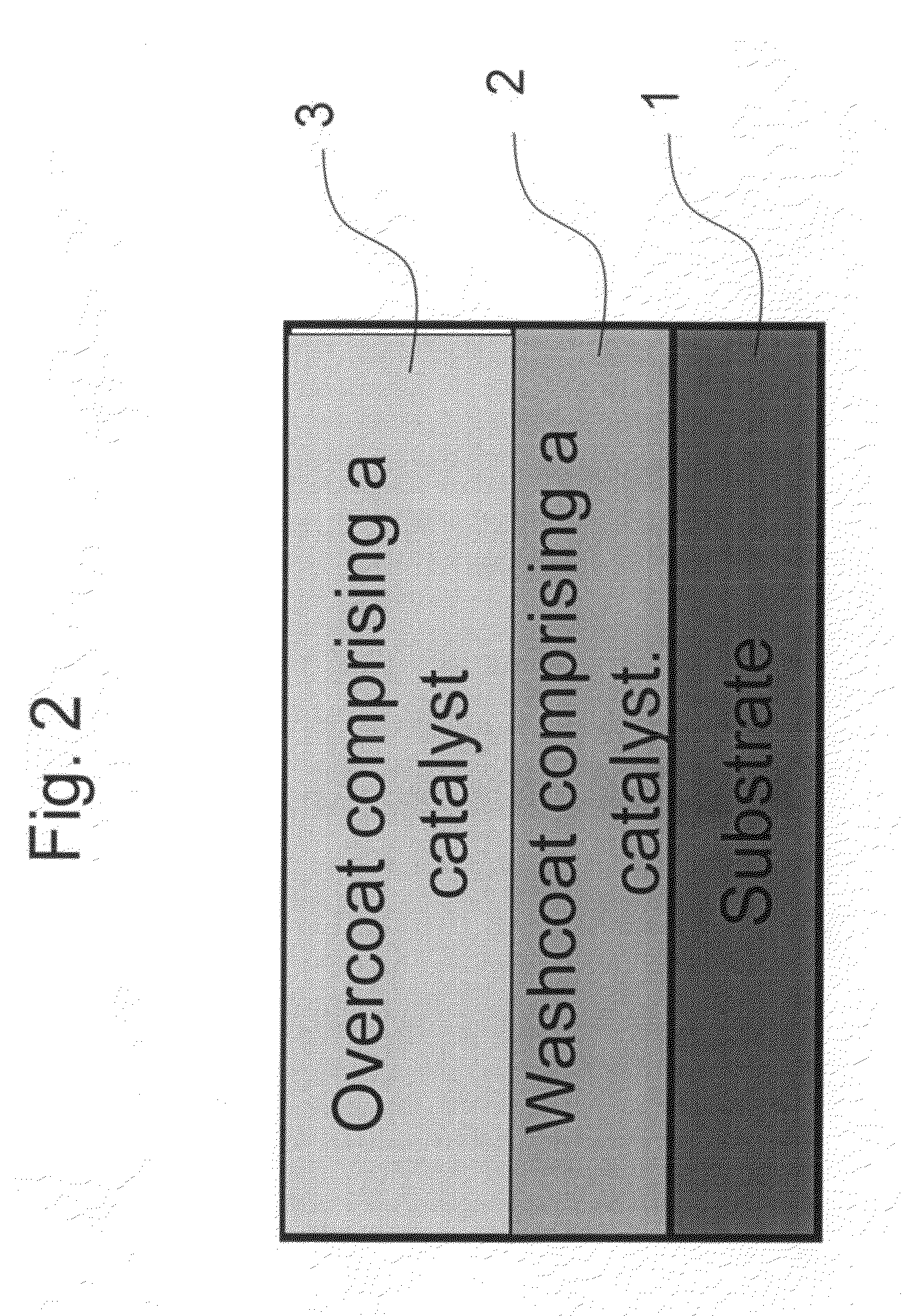
Zero platinum group metal catalysts
a platinum group metal and catalyst technology, applied in the field of catalysts, can solve the problems of driving up the cost of platinum group metals, catalysts and catalytic converters, and achieve the effect of reducing pollutants and reducing pollutants in the exhaus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Pore Volume and Surface Area Measurements for Zero Platinum Group Metal Catalysts
[0123]FIG. 4 shows the measured pore volume for the fresh catalyst systems ZPGM-1 through ZPGM-5 and FIG. 5 shows the measured pore volume for the aged catalyst systems ZPGM-1 through ZPGM-5. The aged catalyst systems were aged at 950° C. for 16 hours with 10% H2O and air. The y-axis on the right side of FIG. 4 is for the pore volume (cm3 / g) of ZPGM-1 only.
[0124]The pore volumes were measured using a Micromeritics® (Norcross, Ga.) TriStar 3000 gas adsorption analyzer at 77K. The pore volumes were obtained from the nitrogen adsorption isotherms using the Barrett-Joiner-Halenda (BJH) method (E. P. Barrett, L. G. Joyner, P. P. Halenda, “The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms,” J. Am. Chem. Soc. (1951), 73, 373-380).
[0125]The results in FIGS. 4 and 5 show that the pore volume decreases for all the catalyst systems (ZPGM-1 through...
example 2
Surface Area Analysis for Fresh and Aged Catalyst Systems ZPGM-1 through ZPGM-5
[0126]The surface areas for the fresh and aged ZPGM catalyst systems are presented in FIG. 6. The aged catalyst systems were aged at 950° C. for 16 hours with 10% H2O and air.
[0127]The surface areas were measured using a Micromeritics® (Norcross, Ga.) TriStar 3000 gas adsorption analyzer at 77K. The surface areas were calculated using the BET (Brunauer, Emmitt and Teller) method (S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309).
[0128]The results in FIG. 6 show that the surface area decreases for all catalyst systems (ZPGM-1 through ZPGM-5) upon aging. The surface area decreases from 18.72 m2 / g for the fresh ZPGM-1 to 2.76 m2 / g for the aged catalyst. Similarly, the surface area decreases from 38.60 m2 / g for the fresh ZPGM-2 to 15.48 m2 / g for the aged catalyst. The surface area decreases from 30.78 m2 / g for the fresh ZPGM-3 to 16.71 m2 / g for the aged catalyst. The surface area decr...
example 3
X-Ray Diffraction Analysis for ZPGM Transition Metal Catalysts
[0129]FIGS. 7-12 show the X-ray diffraction (XRD) patterns of fresh and aged catalyst systems ZPGM-1 through ZPGM-6; the aged catalyst systems were aged at 950° C. for 16 hrs with 10% H2O and air.
[0130]The XRD analysis was conducted to determine the crystalline phases present for each catalyst system. The XRD patterns were measured on a Rigaku® powder diffractometer (MiniFlex™) using Cu Ka radiation in the 2-theta range of 20-70° with a step size of 0.05° and a dwell time of 2 s. The tube voltage and current were set at 40 kV and 30 mA, respectively. The resulting diffraction patterns were analyzed using the International Centre for Diffraction Data (ICDD) database.
[0131]FIG. 7 shows the XRD spectra of the fresh and aged ZPGM-1 catalyst system, Ce0.6La0.4Mn0.6Cu0.4O3, shows the presence of the perovskite (open circles) and fluorite (filled squares) structures. The fluorite and the perovskite structures are larger in the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight ratio | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com