Method For Treating Cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Methods
1.1 Patient Enrolment
[0106]Clinical investigators invited eligible melanoma patients to participate in the trial. The patients were informed about the objectives and risks of the study and were required to sign the consent form before study specific screening procedures were performed. This study was approved by the South Eastern Sydney Area Ethics Committee (00 / 76 Allen) and the NSW Radiation Safety Council and Environmental Protection Authority.
[0107]Criteria for entry into the study were resected patients with documented AJCC / UICC (American Joint Committee on Cancer / International Union against Cancer) stage IV melanoma, at least 18 years of age with expected survival of at least 6 months and adequate haematological, renal and liver functions. Patients had to be able to provide informed consent and to have a Karnofsky Performance Status of at least 70%. Patients were excluded if they had active brain metastasis, active infection or serious medical comorbidity, were ...
example 2a
Intralesional Toxicity
[0130]Enrolment progressed in a stepwise fashion through the planned dose levels. The patients received intralesional injections of the AIC starting from 50 μCi increasing in steps of 100 μCi. The maximum tolerated dose (MTD) was not reached as an effective intralesional dose was obtained at quite low activities. There were no adverse events. In general, the full blood counts and clinical chemistry did not change from the baseline values. There was no significant red cell abnormality nor change in white blood cells and platelets from baseline. Occasional reactive lymphocytes were seen at higher doses. Slight polychromasia was seen in some patients showing fast platelet turnover. Occasional reactive lymphocytes were seen in some patients. The haemoglobin was in the normal range at all doses. There were no significant changes in sodium, albumin and calcium, urea and creatinine at 4 weeks post-TAT. Potassium did not change and was in the normal range for all the p...
example 2b
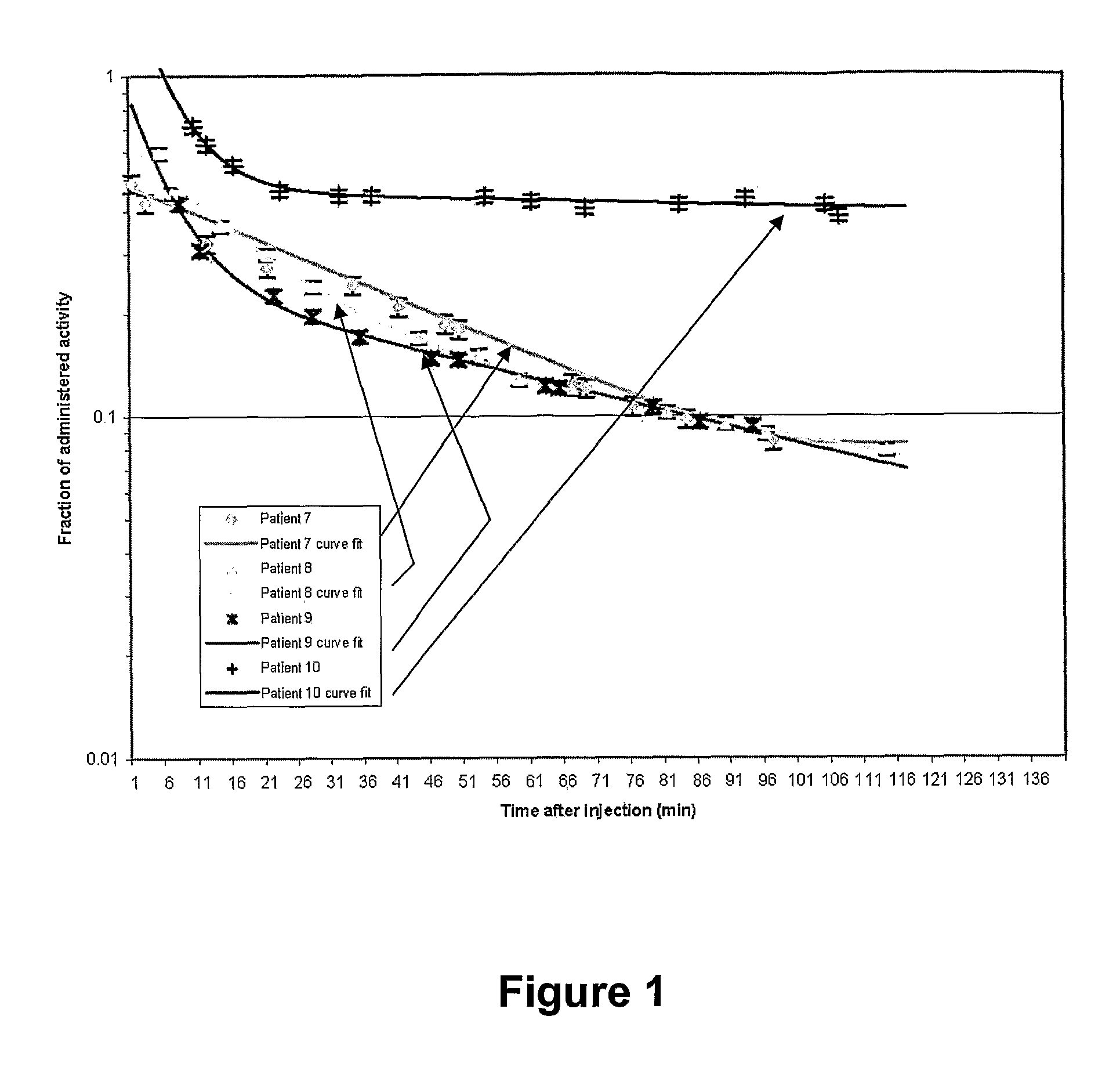
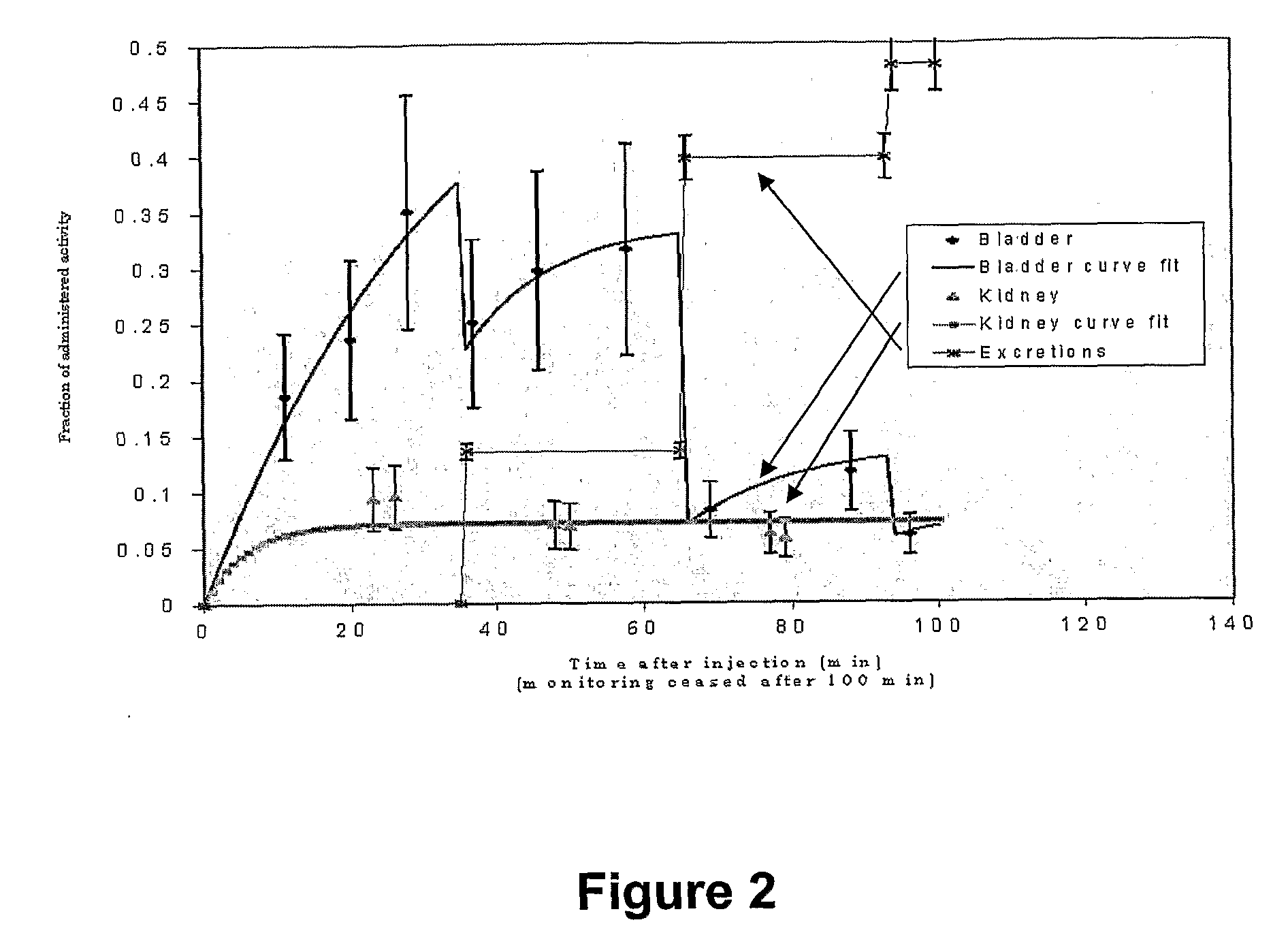
[0132]Kinetics data were corrected for radioactive decay and absorbed dose in the tumour and normal tissues was calculated from the measured activities. Dosimetric analysis was based on measured kinematics using a NaI spectrometer located over the injected tumour, bladder and kidneys as well as urine activity measurements. The bi-exponential fit to the clearance of activity showed rather variable fast and slow clearance rates from the tumour. The mean intensities and exponents were found to be:
Fast clearance0.61 ± 0.090.10 ± 0.02Slow clearance0.16 ± 0.010.03 ± 0.03P value0.00020.003
[0133]These values are significantly different, indicating that the larger fraction of the AIC is cleared rapidly from the tumour, the smaller fraction being retained by receptors on the melanoma cells. Nevertheless, the intralesional AIC was very effective in delivering a high dose to the tumour while sparing other tissues, the absorbed dose being three orders of magnitude higher in the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com