Method of producing lithium ion cathode materials
a lithium ion cathode and lithium ion technology, applied in the field of lithium ion cathode materials, can solve the problems that the heat treatment is not considered suitable for industrial applications, and achieve the effects of enhancing cathode performance, low irreversible capacity, and increasing density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
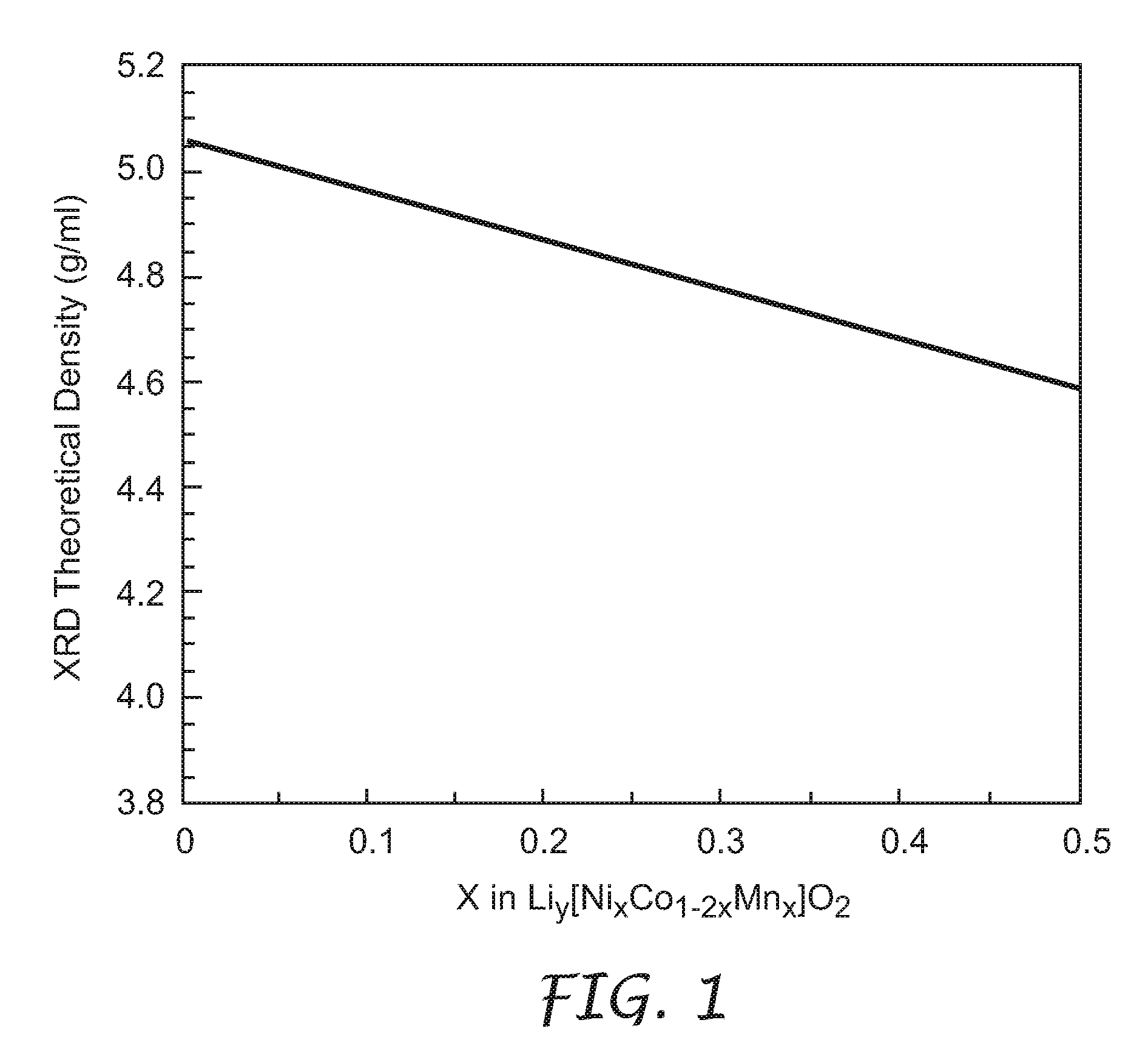
[0030]The lithium metal oxides of the present invention were prepared using the following as starting materials: LiOH.H2O (98%+, Aldrich Chemical Co., Milwaukee, Wis.), CoSO4.7H2O (99%+, Sigma-Aldrich Co. of Highland, Ill.), NiSO4.6H2O (98%, Alfa Aesar, Ward Hill, Mass.), and MnSO4.H2O (Fisher Scientific, Hampton, N.H.). Where not designated, chemicals were obtained from Aldrich Chemical Co., Milwaukee, Wis. All percentages were by weight.
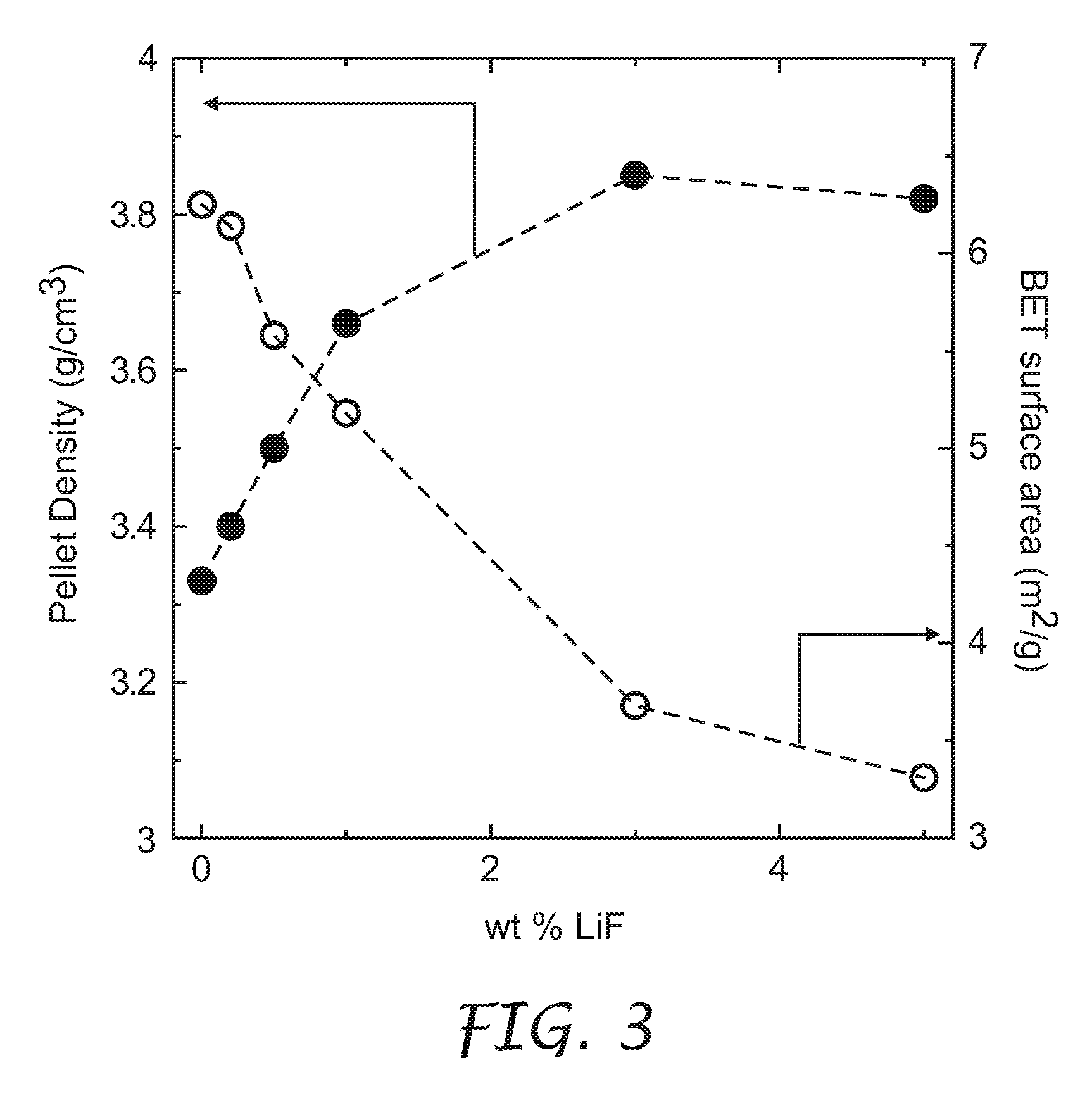
[0031]The process to densify lithium metal oxides of the present invention included two steps. The first step involved a co-precipitation of transition metal sulfate salts in a stirred solution of LiOH to obtain a co-precipitate. It is understood that a solution including any one or more of LiOH, NaOH, and NH4OH can be used as the precipitating agent, leading to the same final improvement in density described herein. The second step comprised mixing the co-precipitate with stoichiometric amounts of Li(OH).H2O and one or both of LiF and B2O3 (both a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com