Lyophilised antigen composition
a technology of lyophilised antigen and composition, which is applied in the direction of drug compositions, immunological disorders, antibody medical ingredients, etc., can solve the problems of increasing the complexity of preparation of components, distribution and formulation of vaccine compositions, and difficulty in liquid vaccine formulations, so as to increase the solubility of lyophilised antigens, increase and improve the solubility of antigens. the effect of cost benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Freeze Drying of a CpG Oligonucleotide and CPC-P501S as Antigen
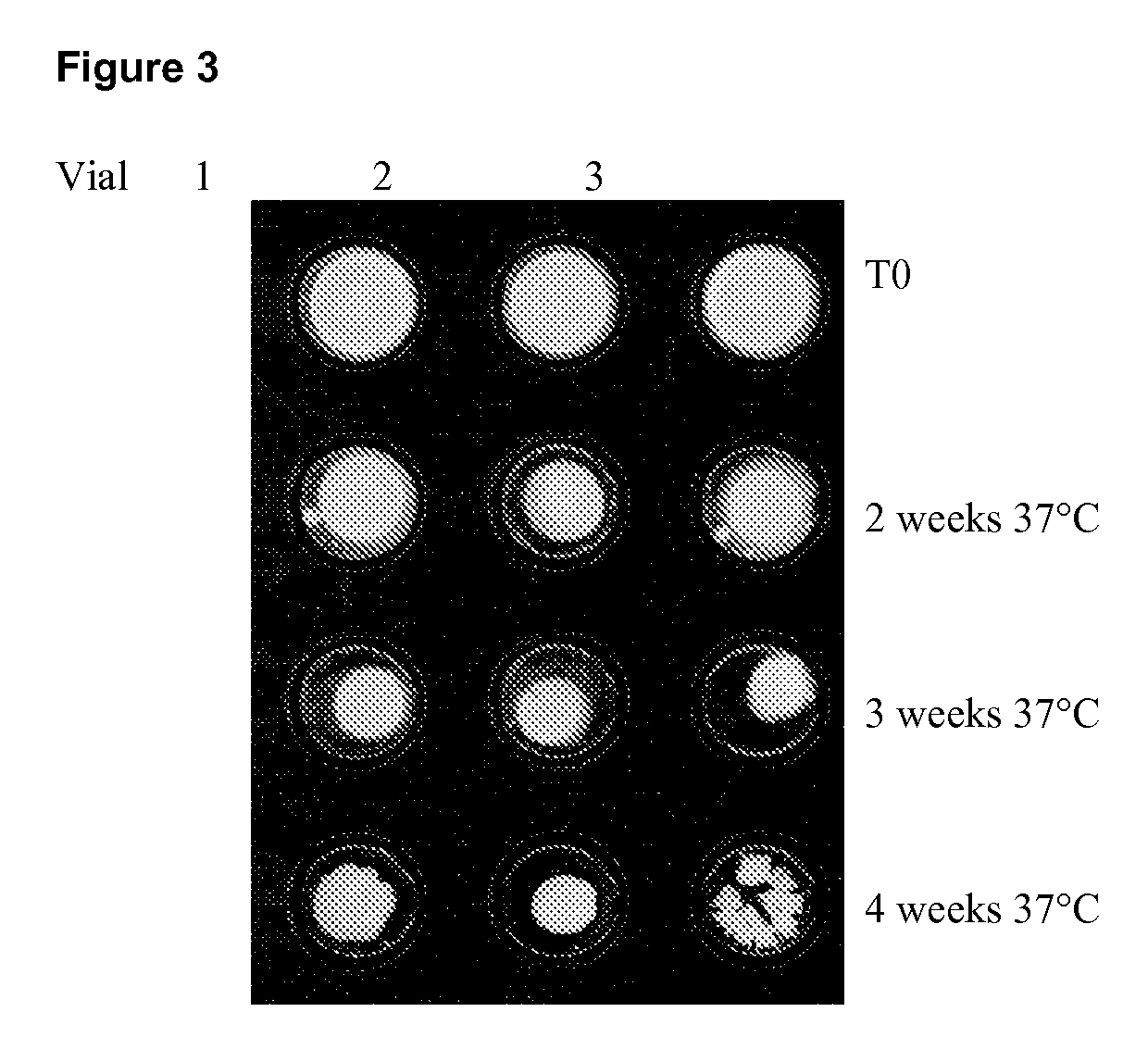
[0159]The antigen used was CPC-P501S. This antigen is shown in FIG. 1 diagrammatically, in which the section showing TM2 to TM12 represents the P501S antigen; the oval shapes on the left hand side represent the CPC fusion partners and the His tail is shown on the right hand side.
[0160]The antigen was produced with a His tag as shown in S. cerevisiae and then made to a concentration of 700 μg / ml using a buffer of Tris (5 mM pH7.5) and Tween80 (0.3%).
[0161]To prepare the final bulk, sucrose (35%) was added to water for injection to reach a final concentration of 6.3%. Tris (1M pH8.8) was then added, followed by Tween 80 (25%) to reach a final concentration of 0.2%. This mixture was magnetically stirred for 5 minutes at room temperature. CPC-P501S was added and the mixture was magnetically stirred for 4 minutes at room temperature. A CpG oligo of SEQ ID No:4 was then added, and the resulting mixture magnetically stirred for...
example 2
Freeze Drying of a CpG Oligonucleotide and Mage-3 as Antigen
[0168]The antigen used was a portion of the protein D protein linked to MAGE-3, which in turn was linked to a His tail for ease of purification PD-Mage3-His (see FIG. 5: SEQ ID NO: 13).
[0169]The purified bulk antigen was produced with a His tag in E. coli and then made to a concentration of 750 μg / ml using a buffer of NaH2PO4.2H2O / K2HPO4.2H2O (2 mM) and Tween80 at approximately 0.2% v / v (theoreitical) pH7.5.
[0170]To prepare the final bulk, sucrose (30%) was added to water for injection to give a final concentration of 3.15%. NaH2PO4.2H2O / K2HPO4.2H2O (100 mM pH7.5) was then added to give a final PO4 concentration of 5 mM taking into account the phosphate found in the antigen buffer. Tween 80 (3%) was also added to give a final concentration of 0.15%, taking into account the Tween found in the antigen buffer. This mixture was magnetically stirred for between 5 and 15 minutes at room temperature. PD-Mage3-His was added (750 μg...
example 3
Impact of CpG on Antigen Solubility Following Reconstitution
[0179]1. WT1 is a protein originally found to be overexpressed in paediatric kidney cancer, Wilm's Tumor. The candidate antigen used in the present case uses nearly the full length protein as antigen. The WT1-A10 protein is a 292 AA recombinant fusion protein expressed in E. coli consisting of a 12mer truncated tat sequence (leader sequence) and amino acids number 2-281 of the WT1 sequence. After lyophilisation alone, this antigen precipates if reconstituted with adjuvant system A due to its isoelectric point (5.85 to 7.5) which is close to the pH of adjuvant system A (6.1) and the presence of sodium chloride in adjuvant system A.
[0180]Two formulations of WT1-A10 were prepared. The reconstituted dose contained 400 μg / ml of WT1-A10 antigen, 10% sucrose, 100 mM Tris, and 0.2% Tween 80, plus or minus 840 μg / ml CpG.
[0181]Both formulations were reconstituted with 500 μl of adjuvant system A. The resulting liquid was centrifuged ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com