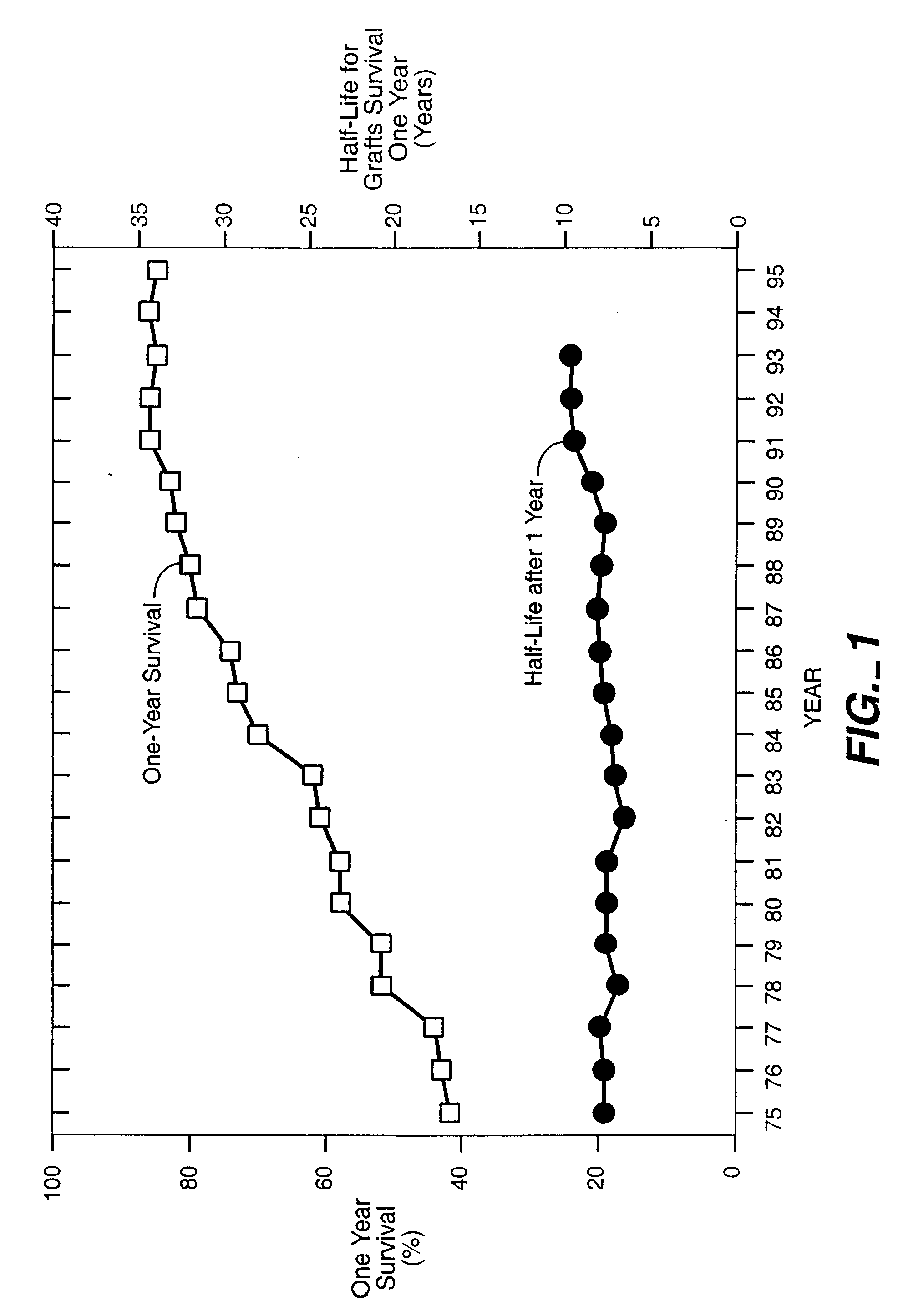
Method to Prevent Graft Rejection Using TGF-Beta to Induce T Suppressor Cells
a technology of tgfbeta and t suppressor cells, which is applied in the direction of antibody medical ingredients, drug compositions, immunological disorders, etc., can solve the problems of lack of progress in preventing chronic allograft rejection, significant side effects of long-term immunosuppressive therapy, and less success of drugs, so as to reduce the probability of serious side effects, inhibit cytotoxic activity, and prevent the lysis of donor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
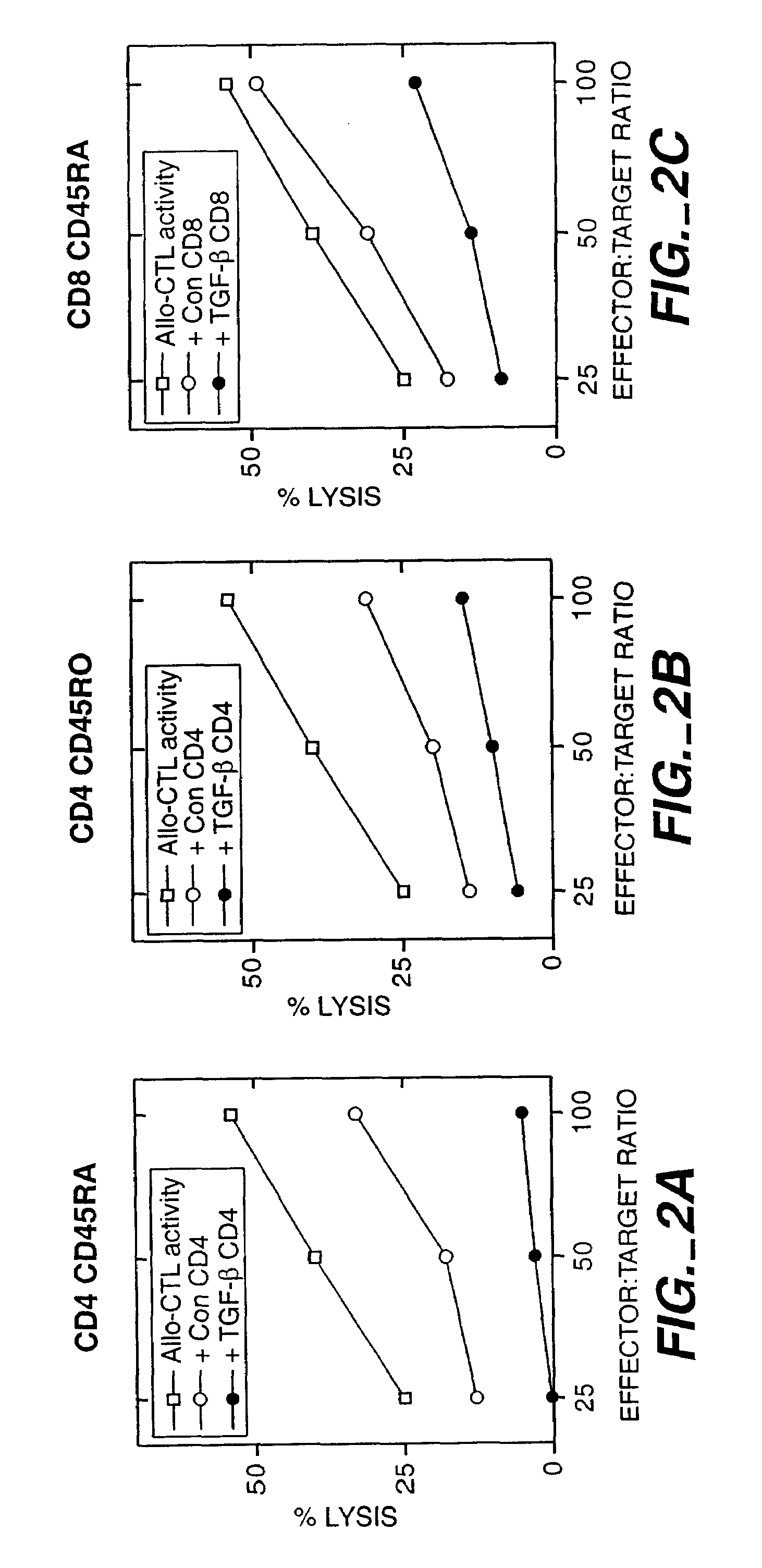
TGF-β Activated CD4+ T Cells Suppress Cytotoxic T Cell Activity
[0071]Blood from both donor and recipient were obtained by pheresis. PBMC from each individual were separated from RBC using the Nexell Isolex 500 closed system. CD4+ cells from the recipient and T cell-depleted mononuclear cells from the donor were prepared using commercially available reagents.
[0072]If the recipient receives an organ transplant from a donor whose mononuclear cells are not available, the recipient's T cells are conditioned with irradiated mononuclear cells from a pool of donors which express a broad panel of common and uncommon histocompatibility antigens.
[0073]Recipient CD4+ cells were cultured with irradiated donor mononuclear cells in the presence of TGF-β for 5 days. The CD4+ cells which react with donor cells in the presence of TGF-β were induced to become suppressor T cells. The cells were incubated with donor alloantigens or mitogens for an additional 10 days to expand the number of suppressor T ...
example 2
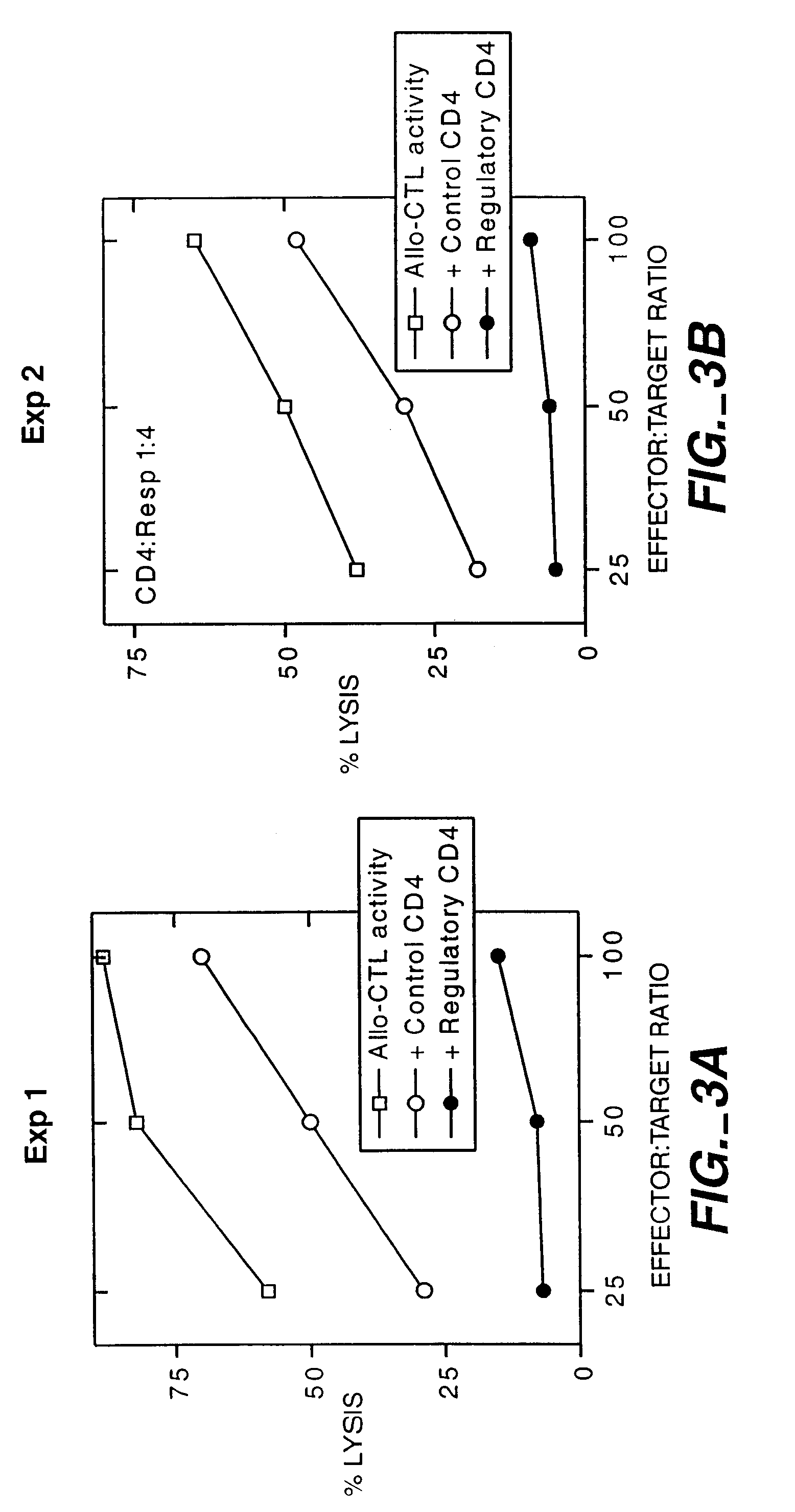
TGF-β Activated T Cells Suppress Cytotoxic T Cell Activity
[0076]Blood from both donor and recipient were obtained by pheresis. PBMC from each individual were separated from RBC using the Nexell Isolex 500 closed system. T cells from the recipient which contain both the major subsets (CD4+ and CD8+ cells) and the minor subsets (TNK cells and gamma delta T cells), and T cell-depleted mononuclear cells from the donor were prepared using commercially available reagents.
[0077]Recipient T cells were cultured with irradiated donor mononuclear cells in the presence of TGF-β for 3 to 5 days. The various T cell subsets which react with donor cells in the presence of TGF-β were induced to become suppressor T cells. Similar procedures were repeated for an additional 10 days to expand the number of suppressor T cells.
[0078]The recipient's T cells primed with donor alloantigens in the presence of TGF-beta are then tested for suppressive activity by showing that they prevent recipient's precursor ...
example 3
Treatment of T Cells from a Recipient of Kidney Graft from a Non-Identical Sibling Donor to Prevent Graft Rejection
[0079]CD4+ cells conditioned with 1 ng / ml TGF-β, are transferred to the recipient 1 day before kidney transplant and allowed to “home” to lymphoid tissue. These CD4+ cells circulate to the recipient's lymphoid organs, where they block the recipient's T cell response to donor histocompatibility antigens. As a result, the recipient's T cells become tolerant to the donor's histocompatibility antigens. This tolerance reduces acute rejection, lessening the need for high doses of immunosuppressive drugs. As the recipient's lymphocytes are “educated” to develop long lasting tolerance, chronic rejection is decreased or eliminated. If signs of graft rejection recur, additional infusions of regulatory T cells will ameliorate this response.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com