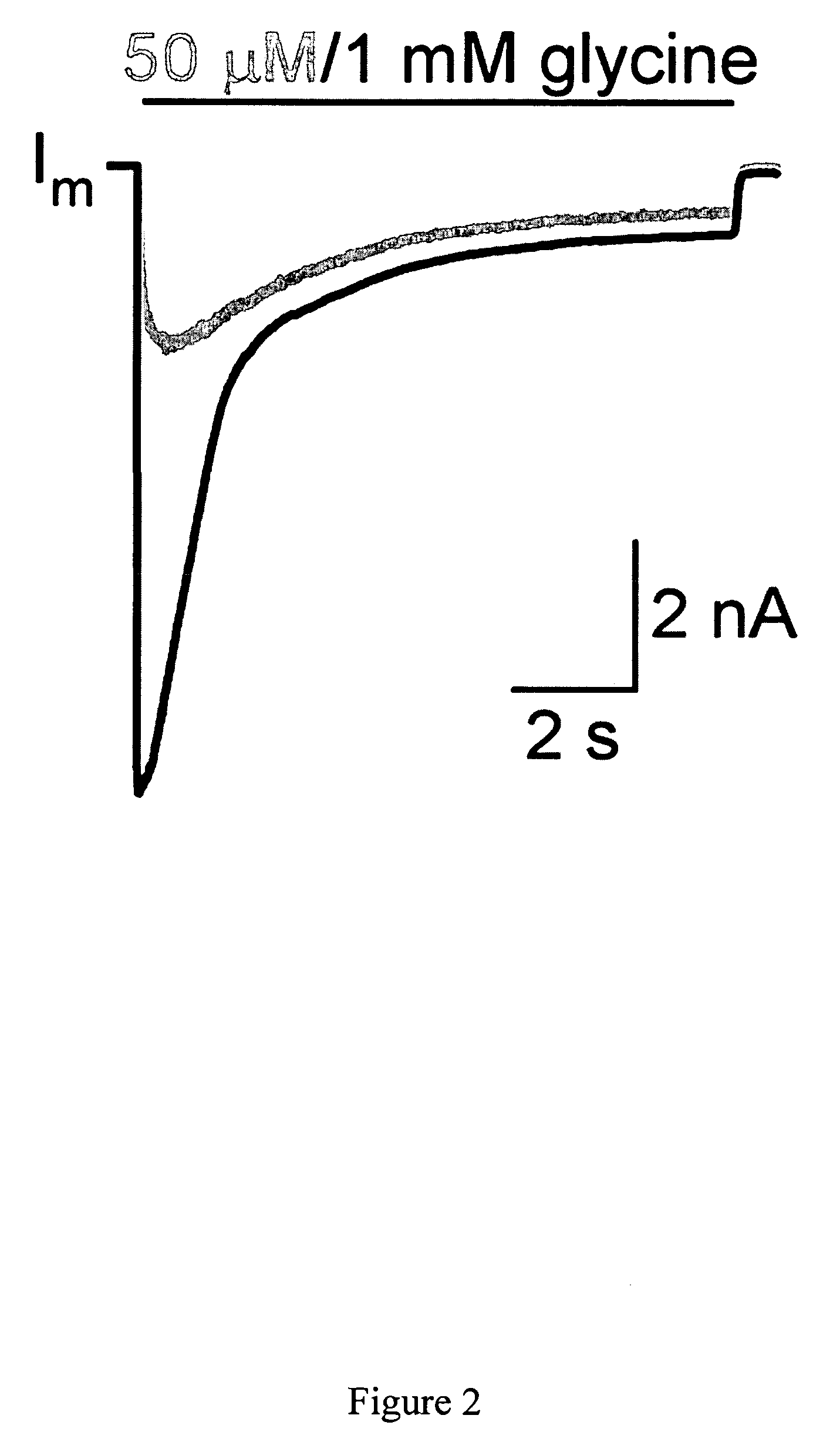Targeted delivery of glycine receptors to excitable cells
a glycine receptor and excitable cell technology, applied in the direction of dsdna viruses, peptide/protein ingredients, drug compositions, etc., can solve the problems of affecting the normal function of excitable cells, and affecting the ability of excitable cells to function normally, so as to reduce formalin-induced nociceptive behavior, and alleviate observed pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0041]This example demonstrates targeted delivery of glycine receptors to peripheral neurons as treatment for pain.
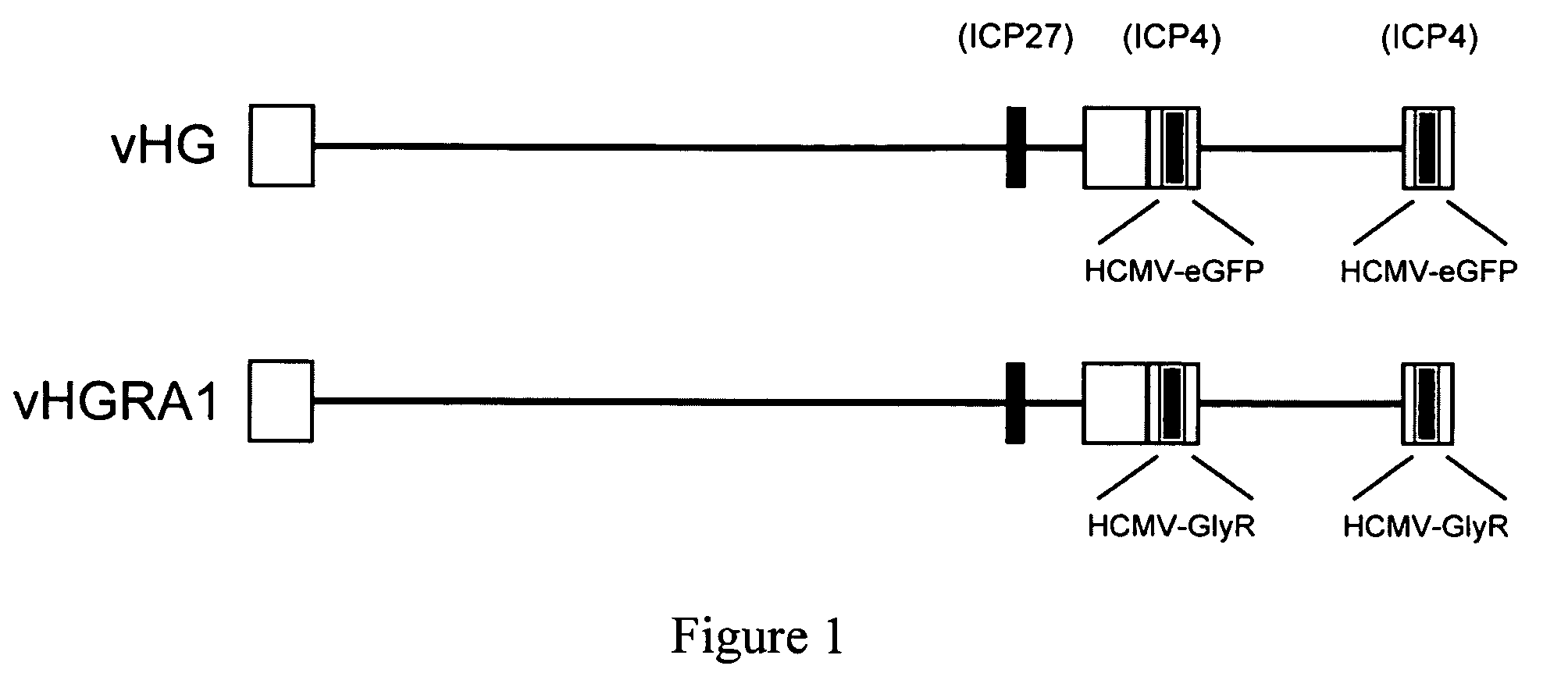
[0042]Production of recombinant virus. The plasmid pSHB2-GlyR was created by cloning the GlyR sequences into plasmid SHB2 at the BamHI site of the polylinker. SHB2 was generated by ligation of a HCMV-BGHpA expression construct from pRC-CMV into plasmid pSASB3 at the unique BamHI site. pSASB3 was constructed by cloning the Sph I to Afl III (Sal I linkered) fragment (1928 bp) of the HSV-1 KOS strain genome (nucleotides 124485-126413) into Sph I / Sal I digested pSP72 followed by insertion of a the 695 bp BglII to BamHI fragment (nucleotides 131931 to 132626) containing regions upstream of the ICP4 promoter including the viral origin contained within the short inverted repeat regions into the BglII to BamHI sites of the vector plasmid. The parental virus vHG was created using the same targeting plasmid except that a HCMV-eGFP construct was cloned into the same BamHI site res...
example 2
[0052]This example demonstrates a method for inhibiting pain in vivo comprising exogenously expressing a GlyR protein.
[0053]The analgesic efficacy of vHGlyRα1 will be assessed in three mouse pain models: (1) the formalin footpad model of acute inflammatory pain, (2) the complete Freund's adjuvant (CFA) model of chronic inflammatory pain, (3) spinal nerve ligation model, (4) the chronic constriction injury (CCI) model of chronic neuropathic pain, and (5) osteolytic sarcoma model of bone cancer pain. Each of these pain models is described in detail below.
[0054]Formalin footpad test (FFT): This is an acute model of inflammatory pain (Dubisson and Dennis, Pain, 4(2): 161-74 (1977)). 50 μl of 2.5% formalin will be injected subcutaneously into the plantar surface of the right hind foot. This will cause acute pain lasting approximately 1-2 hours. Following formalin injection, each animal will be placed in a clear rectangular plexiglass cage (20 cm wide×60 cm long×20 cm high). Different pos...
example 3
[0064]This example demonstrates a method of generating a transgenic animal that exogenously expresses a GlyR protein.
[0065]A cb-stop-lacZ construct (Zinyk et al., Curr Biol., 8(11):665-8 (1998)) will serve as the base construct for production of transgenic mice. This construct contains (5′ to 3′) the constitutively active chicken β-actin promoter and the first two non-coding exons, a transcription / translation stop cassette that is flanked by loxP sequences, a lacZ reporter cassette, and the 3′ untranslated region / polyadenylation site from the mouse protamine-1 gene. In previous studies of transgenic mice that harbor this construct (see, e.g., Zinyk et al., supra), it was demonstrated that in the absence of Cre-mediated recombination, the reporter cassette was inactive. Following Cre-mediated recombination and deletion of the stop cassette, the reporter cassette was expressed.
[0066]Standard molecular techniques will be used to replace the lacZ reporter with either a wild-type or a mu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Attenuation coefficient | aaaaa | aaaaa |
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com