Hydrotalcite-Like Substance, Process for Producing the Same and Method of Immobilizing Hazardous Substance
a technology of hydrotalcite and similarity, applied in the direction of ion-exchangers, nuclear engineering, transportation and packaging, etc., can solve the problem that the conventional ones cannot achieve the desired effect of anionic hazardous substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first example
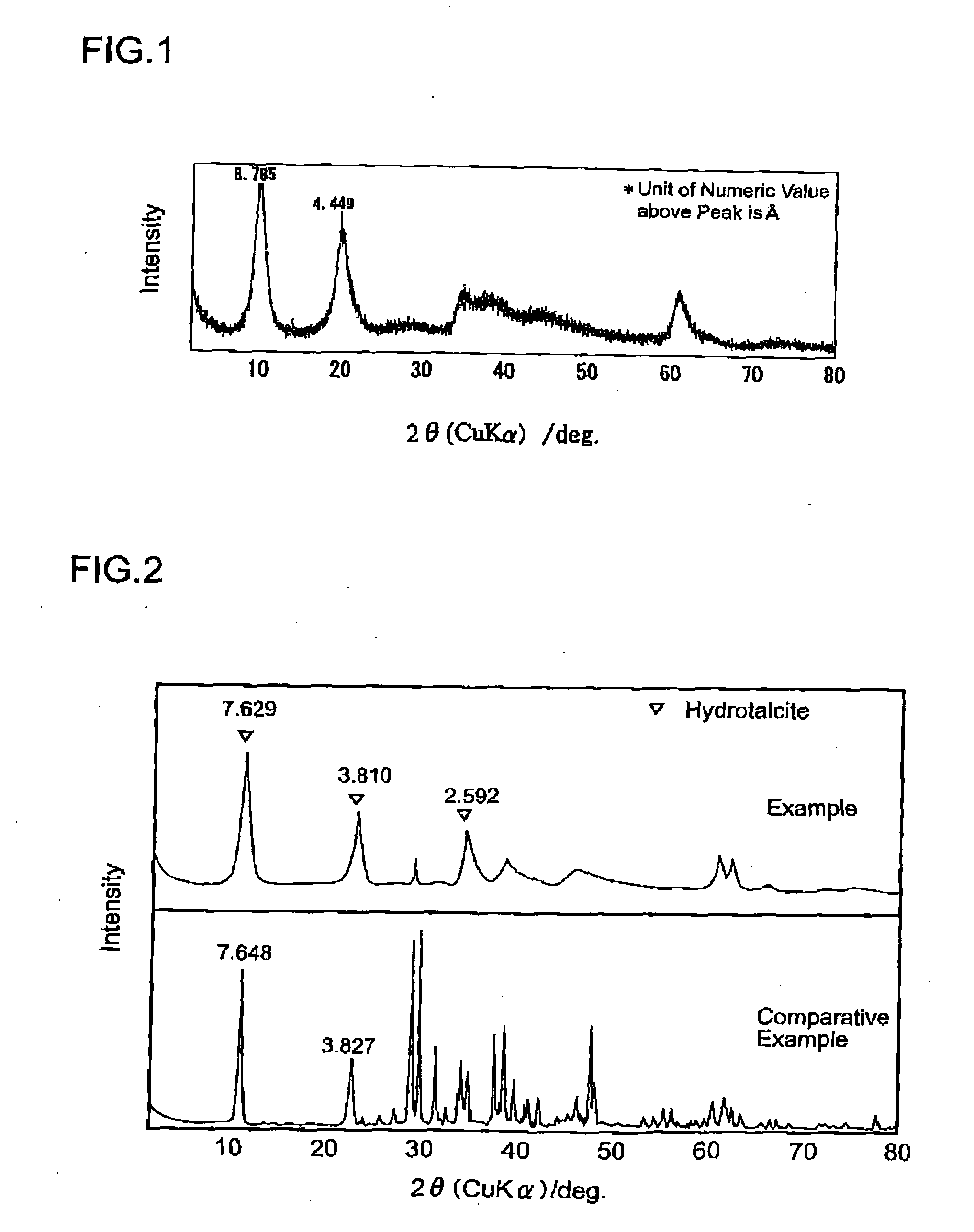
[0047]A hydrotalcite-like substance and a process for producing the same in accordance with an example of the present invention will be explained hereafter. Although temperature is kept at 80 degrees C. or less in all the processes in this example, temperature conditions are not limited thereto, but should just be about 100 degrees C. or lower.
[0048]First, in order to produce a hydrotalcite-like substance of the present invention, an acidic solution containing aluminum ions and magnesium ions are prepared.
[0049]As for an aluminum source of aluminum ions, it is not limited to a specific substance as long as it can generate aluminum ions underwater. For example, alumina, sodium aluminate, aluminum hydroxide, aluminum chloride, aluminum nitrate, bauxite, residue left after producing alumina from bauxite, aluminum sludge, etc. may be used. These aluminum sources may be used either independently or in combination with one or more other sources.
[0050]As for a magnesium source of magnesium...
second example
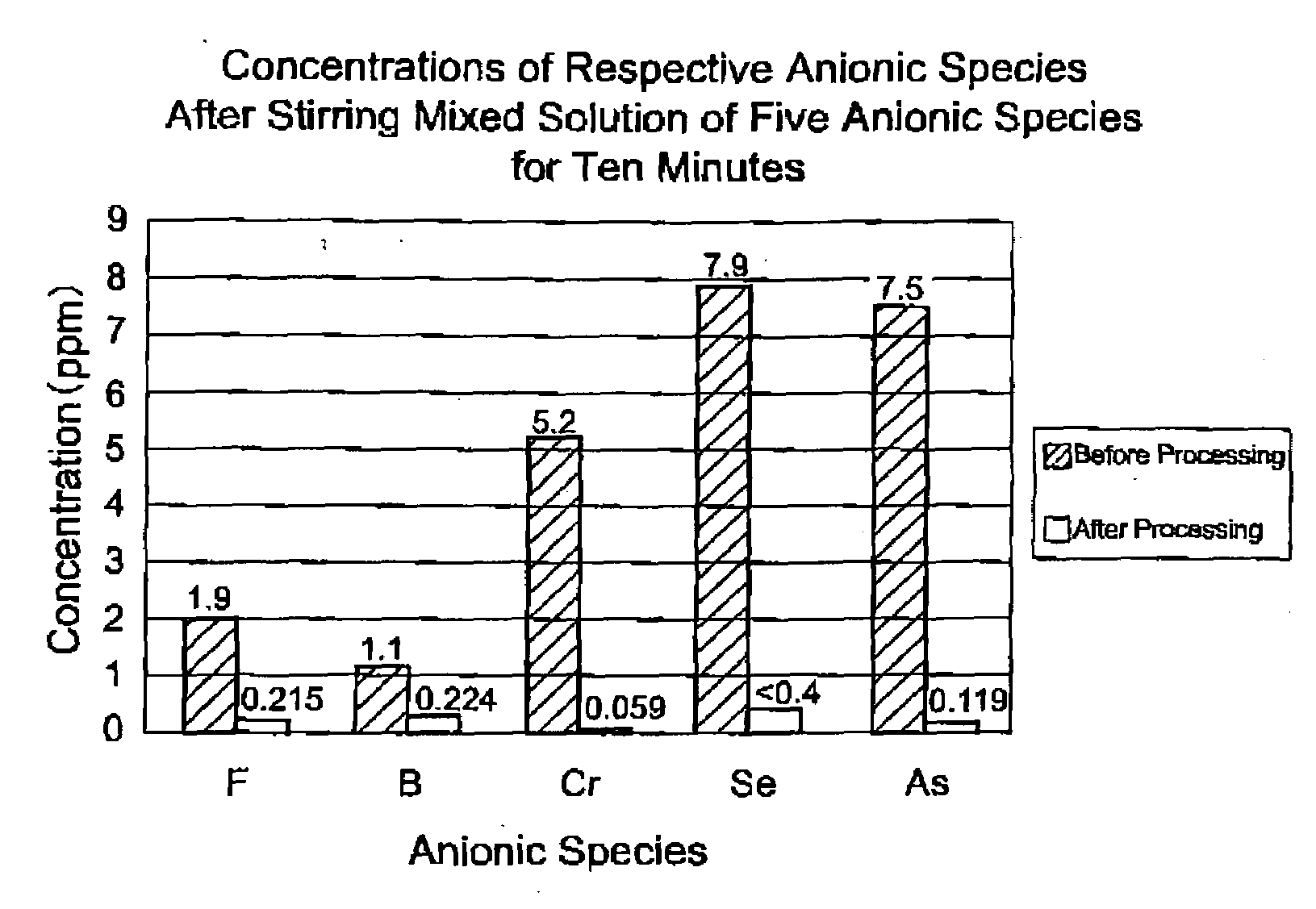
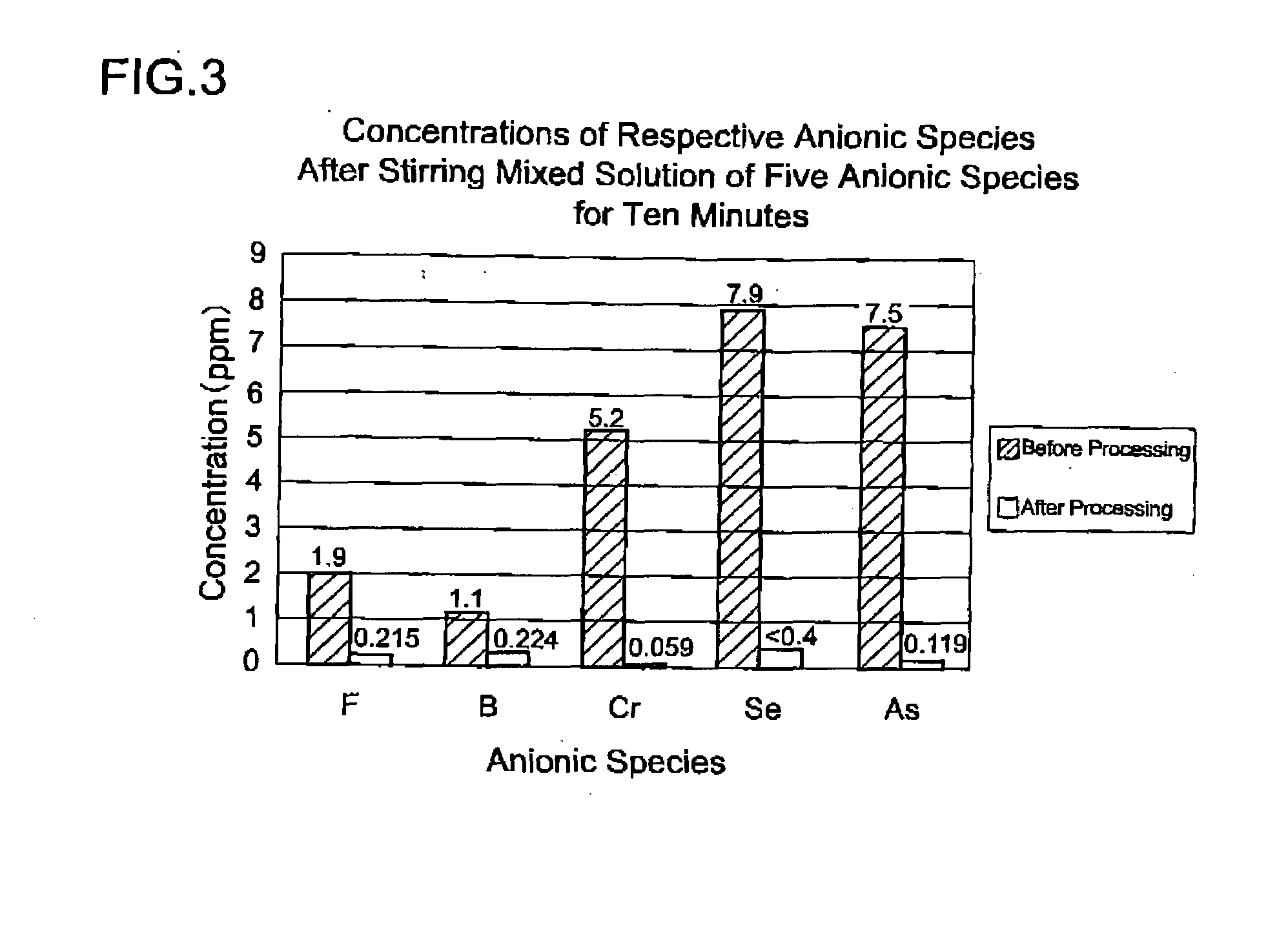
[0094]Next is a description of a method of immobilizing a hazardous substance using the hydrotalcite-like substance obtained in the foregoing example.
[0095]The hydrotalcite-like substance is used in such a manner that if it is in a slurry form obtained by for example dispersing in water, it is pushed out toward the target object containing a hazardous substance, using a manual or a pressure pump, or others means. Alternatively, the hydrotalcite-like substance that is dried and reduced to powder may be used in such a manner that it is pushed out toward the target object containing a hazardous substance, using a manual or a pressure pump, or others means.
[0096]As described above, the present example of the invention is such that a hydrotalcite-like substance is synthesized by mixing the acidic solution containing aluminum ions and magnesium ions with the alkaline solution containing alkali, and then it is subjected to water removal or neutralization process without ageing; and the hyd...
third example
[0099]The method of immobilizing a hazardous substance in accordance with the third example of the invention proposes pour the acidic solution containing aluminum ions and magnesium ions used in the foregoing examples into a target object while mixing the same with alkali.
[0100]By pouring the acidic solution while mixing it with alkali anions of the hazardous substance can be immobilized due to anion exchange that takes place in the production process of the hydrotalcite-like substance. By immobilizing the anions of the hazardous substance in the production process of the hydrotalcite-like substance thus way, more efficient immobilization of the hazardous substance can be achieved as compared to a case where the hydrotalcite-like substance synthesized beforehand or a powder thereof is added.
[0101]The method according to the present example is applicable to a target object containing hazardous substances, such as soil that has already underwent solidification treatment with cement et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallite size | aaaaa | aaaaa |
| crystallite size | aaaaa | aaaaa |
| grain size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com