Degradable Cage Coated With Mineral Layers For Spinal Interbody Fusion
a technology of interbody fusion and cages, applied in the field of cages, can solve the problems of reducing the efficacy of interbody fusion, increasing the incidence of postoperative complications, and synovitis and the lymphatic spread of non-absorbable polymer debris, etc., to achieve superior binding capacity, prolong the life of the implant, and enhance the bioactivity of the spinal implan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
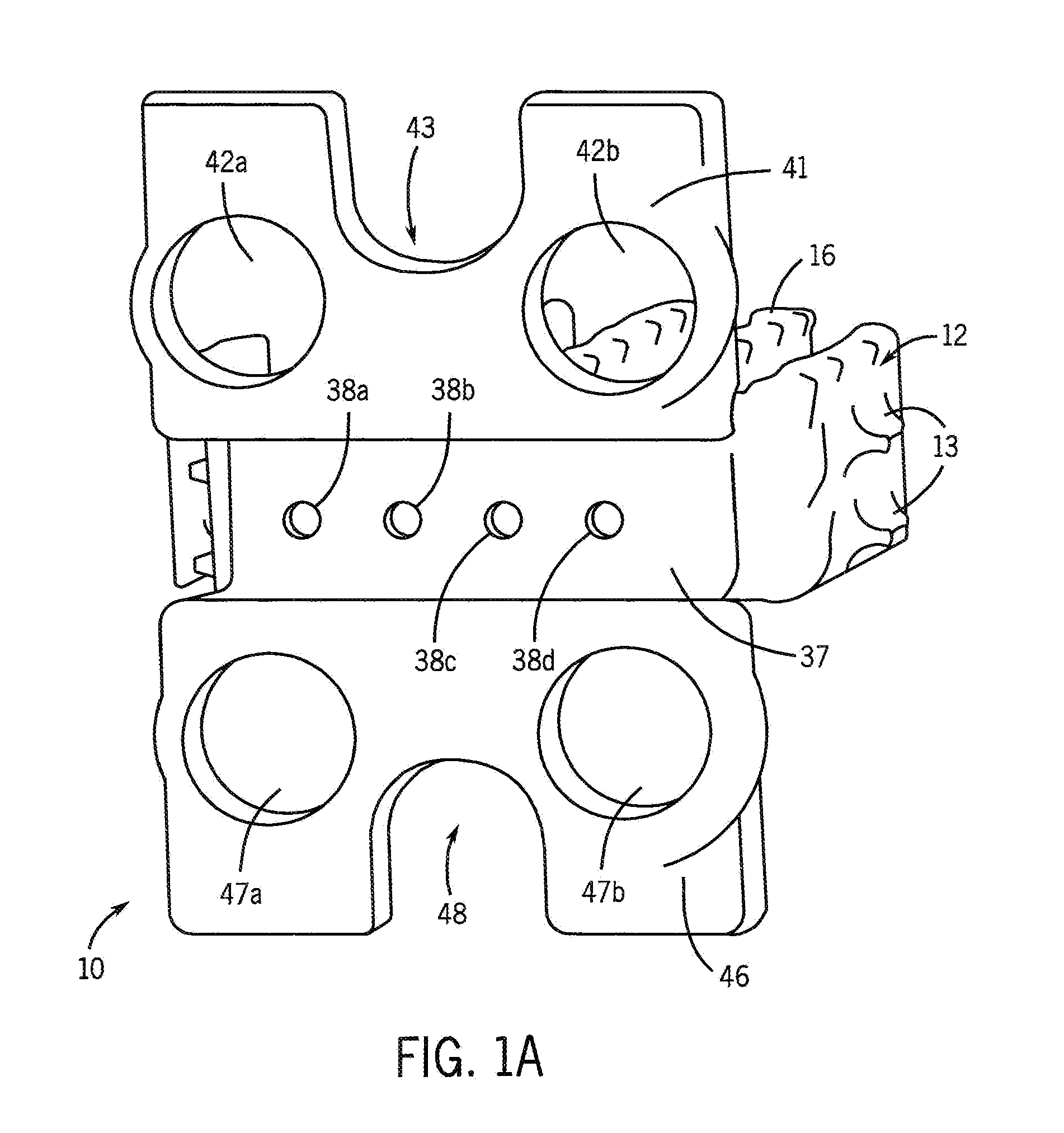
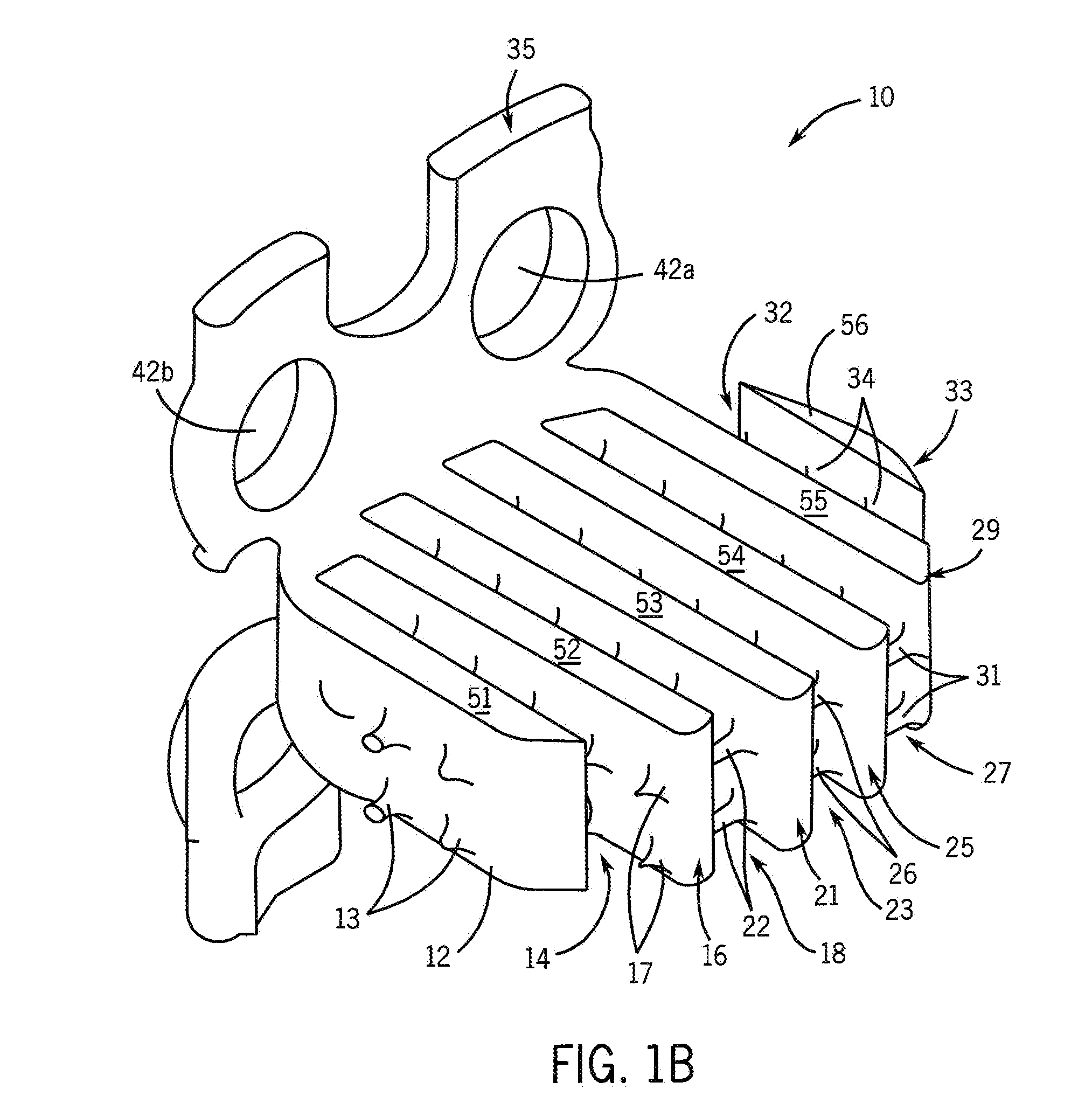
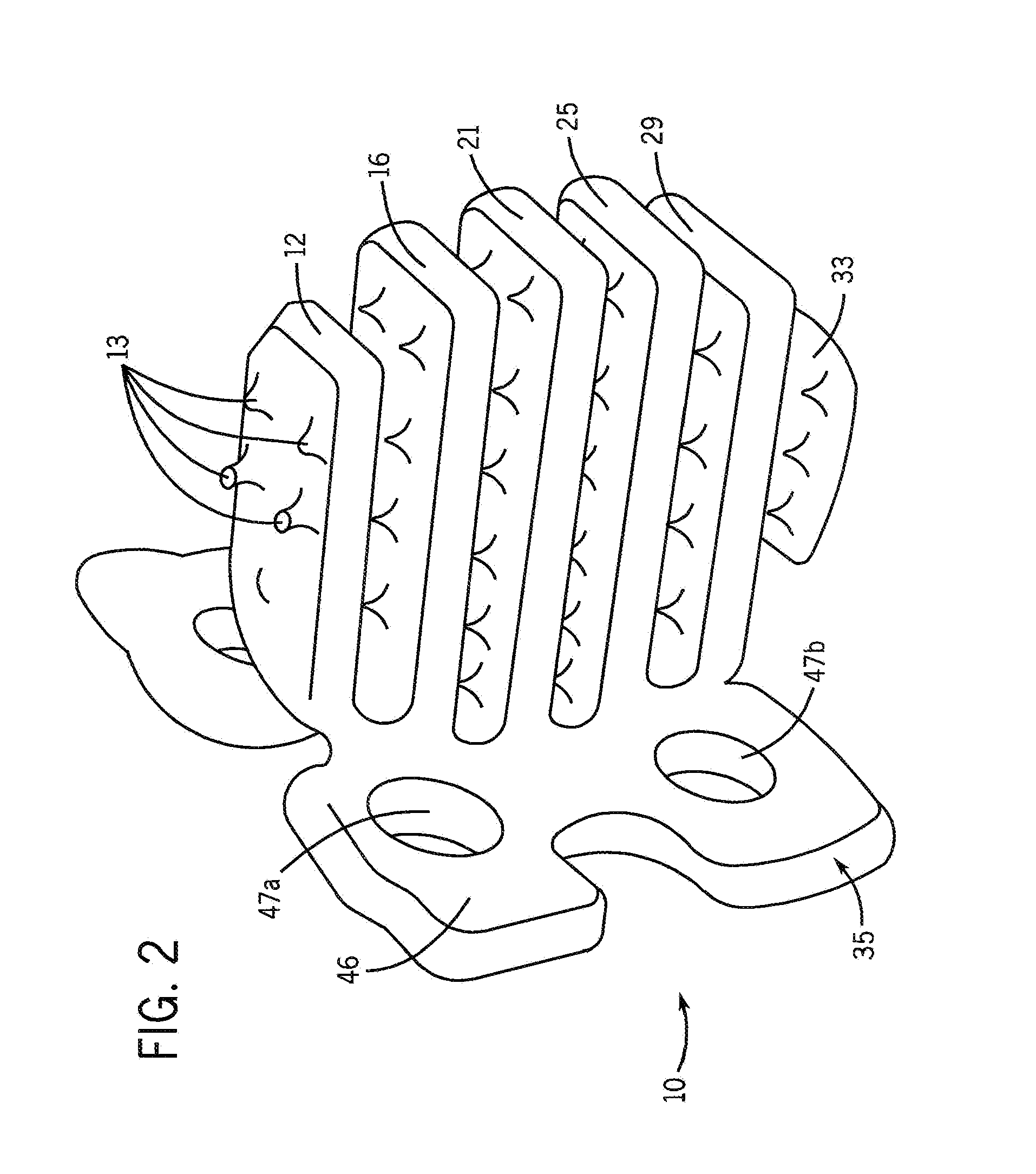
[0028]One purpose of the proposed invention is to develop a simple and flexible method that enhances osteogenesis to achieve spine arthrodesis induced by biologically active bone morphogenetic proteins released from osteoconductive, biodegradable spine fusion cages. The working hypothesis of these studies is that bone morphogenetic proteins incorporated in a calcium phosphate mineral coating on a polycaprolactone spine fusion cage will induce more rapid and complete bone regeneration when compared with bone morphogenetic proteins delivered from a collagen sponge placed within a cage. The approach we use to grow calcium phosphate mineral coatings on polycaprolactone cages is a low temperature process, which will allow for incorporation of active bone morphogenetic proteins after mineral growth via surface binding. The resulting composite cage will then contain biologically active growth factors, which will be released upon mineral dissolution and / or degradation of the cage. The degra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Young's modulus | aaaaa | aaaaa |
| Young's modulus | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com