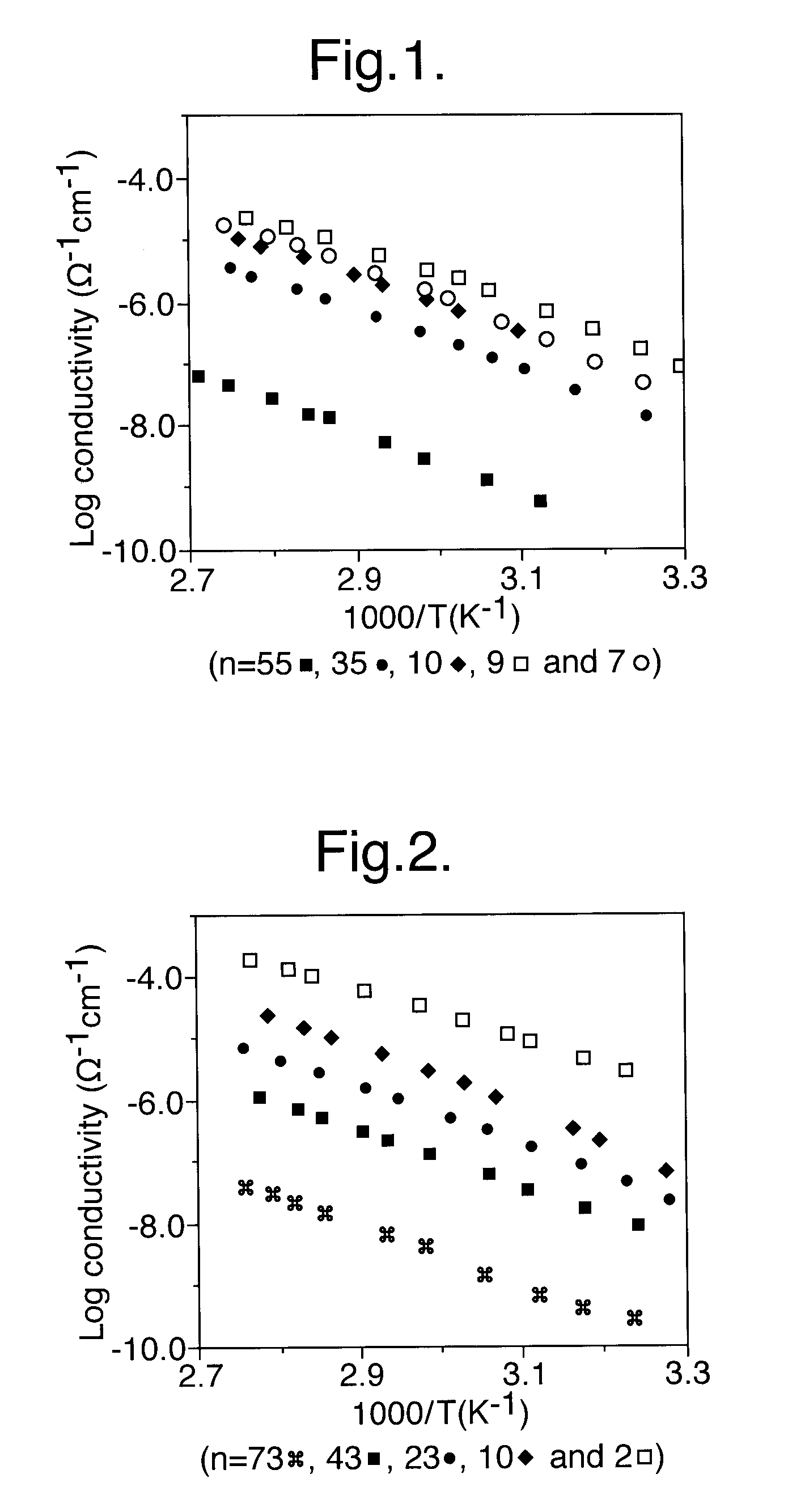
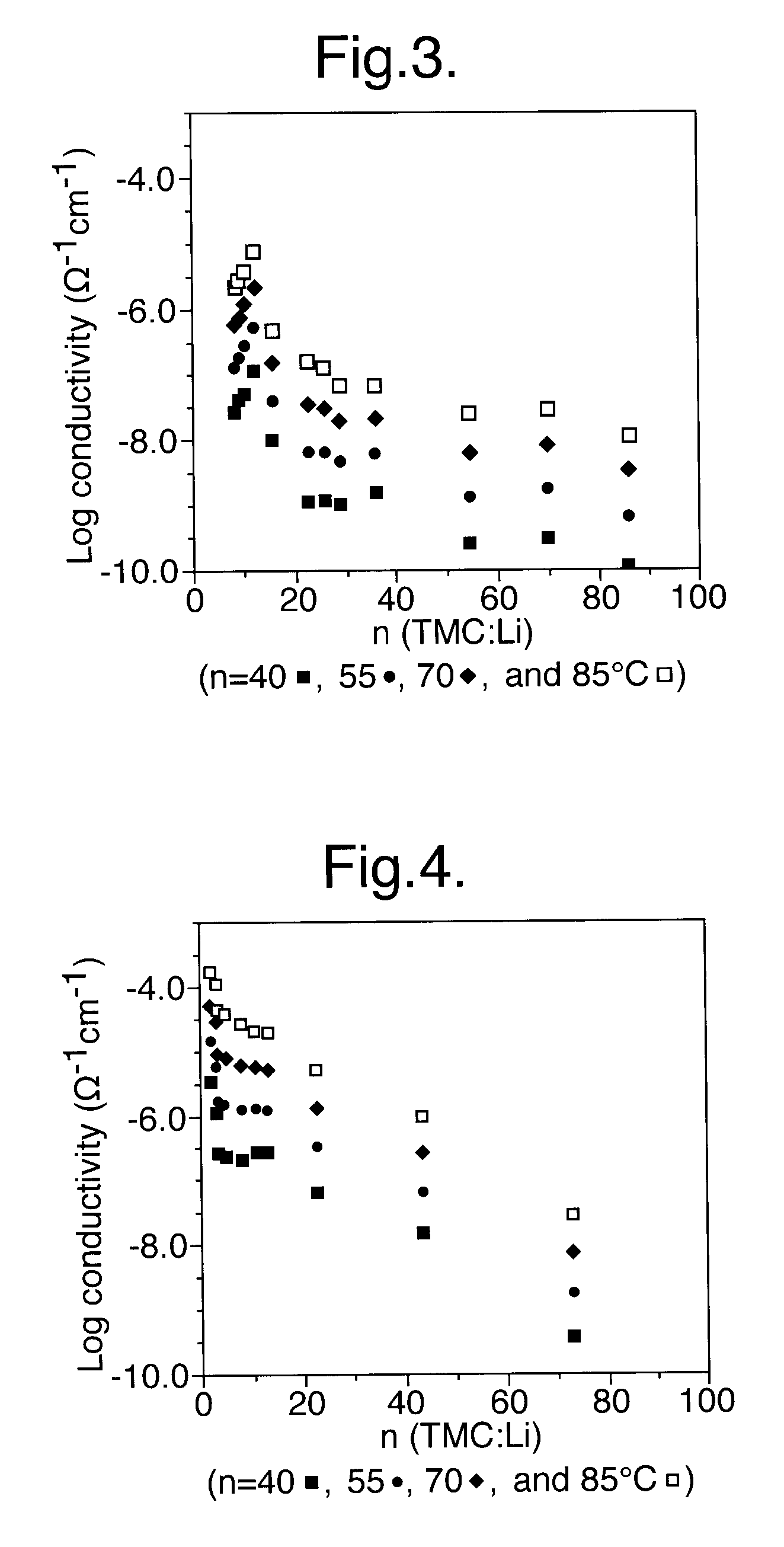
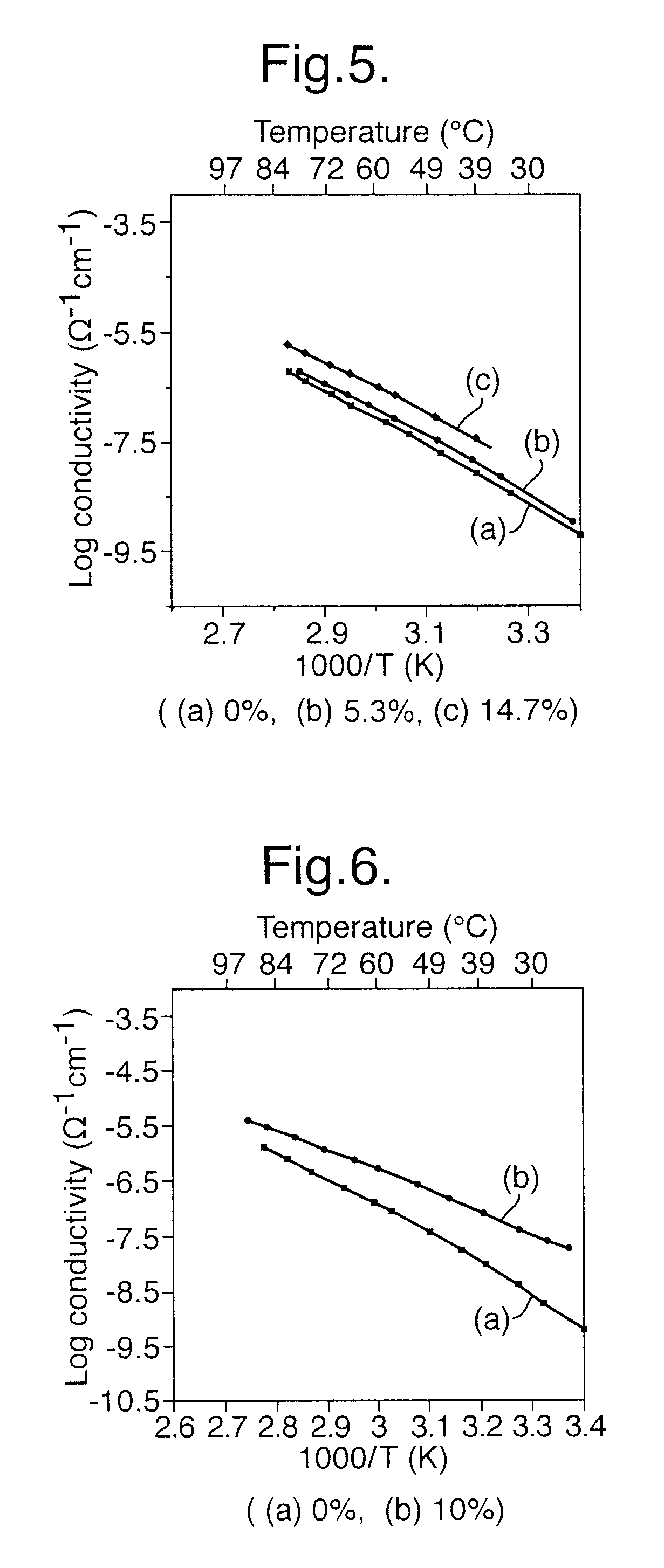
Method for preparing composition for use as a polymer electrolyte
a polymer electrolyte and composition technology, applied in the field of preparation of compositions for use as polymer electrolyte, can solve the problems of liquid electrolyte leakage, poor mechanical compatibility with certain types of electrode materials, and low ionic conductivity, and achieve good ionic conductivity and excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045]By “polar materials” in the present invention is meant salts and proton conductor systems. Typical proton conductor systems include phosphoric acid and sulphamide.
[0046]By “hydrocarbyl group” in the present invention is meant a group containing only carbon and hydrogen. Unless otherwise stated, the number of carbon atoms is preferably from 1 to 4. Examples of hydrocarbyl groups include methyl, ethyl and propyl.
[0047]In the present invention, the phrase “optionally substituted hydrocarbyl” is used to describe hydrocarbyl groups optionally containing one or more “inert” heteroatom-containing functional groups. By “inert” is meant that the functional groups do not interfere to any substantial degree with the ionic conductivity and / or mechanical properties of the resulting composition. Said inert groups may include ethers and polyethers. Such inert groups may act as plasticisers. Non-limiting examples of such inert groups are —CH2CH2OCH3 and —CH2CH2OCH2CH2OCH3.
[0048]Inert function...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tg | aaaaa | aaaaa |
| Tg | aaaaa | aaaaa |
| Tg | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com