Bioengineered Tissue Constructs and Methods for Producing and Using Thereof
a bioengineered tissue and construct technology, applied in the field of tissue engineering, can solve the problems of scientific challenges in creating new bioengineered tissue, and achieve the effect of easy and peeling removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formation of a Collagenous Matrix by Human Neonatal Foreskin Fibroblasts
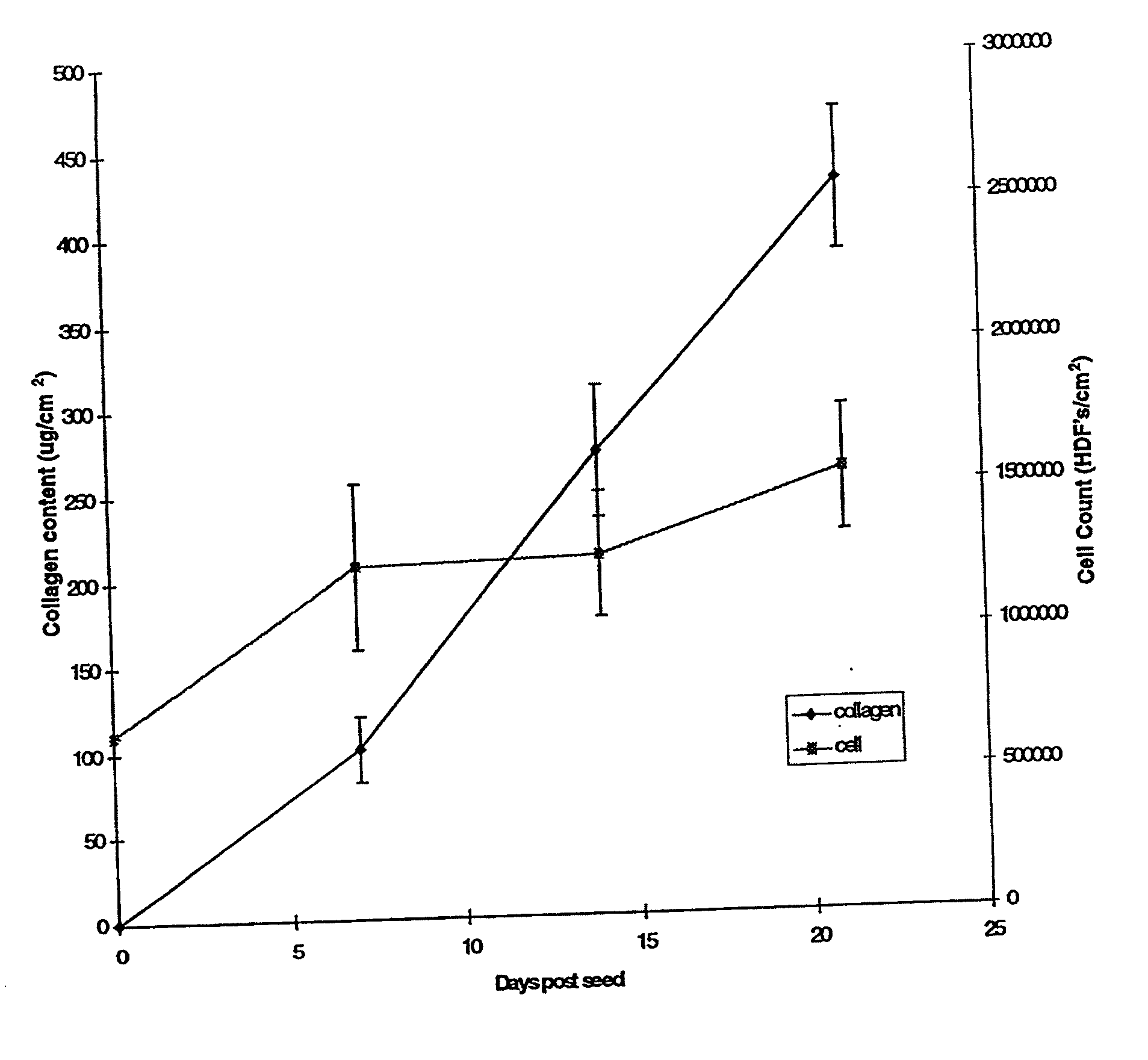
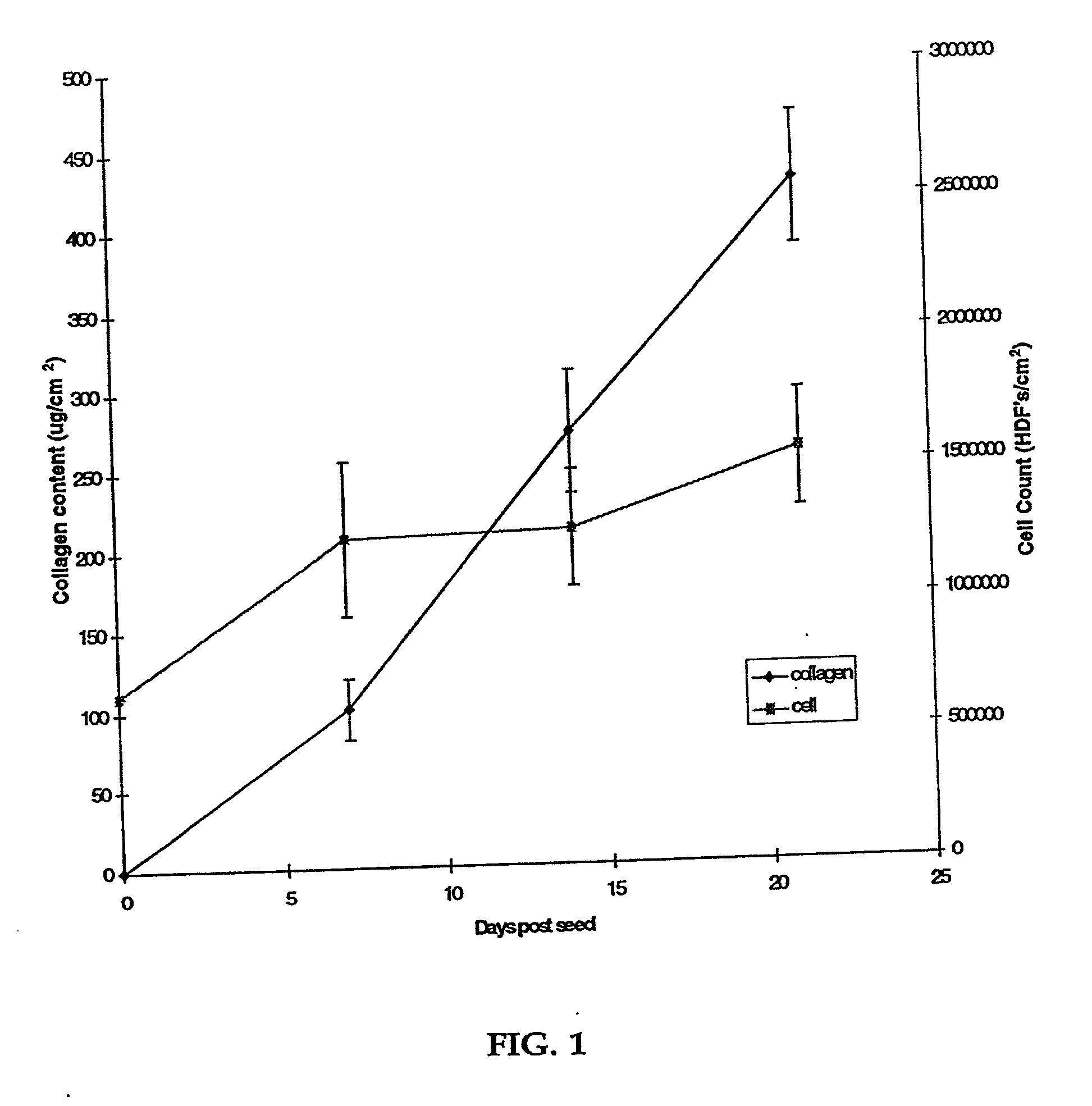
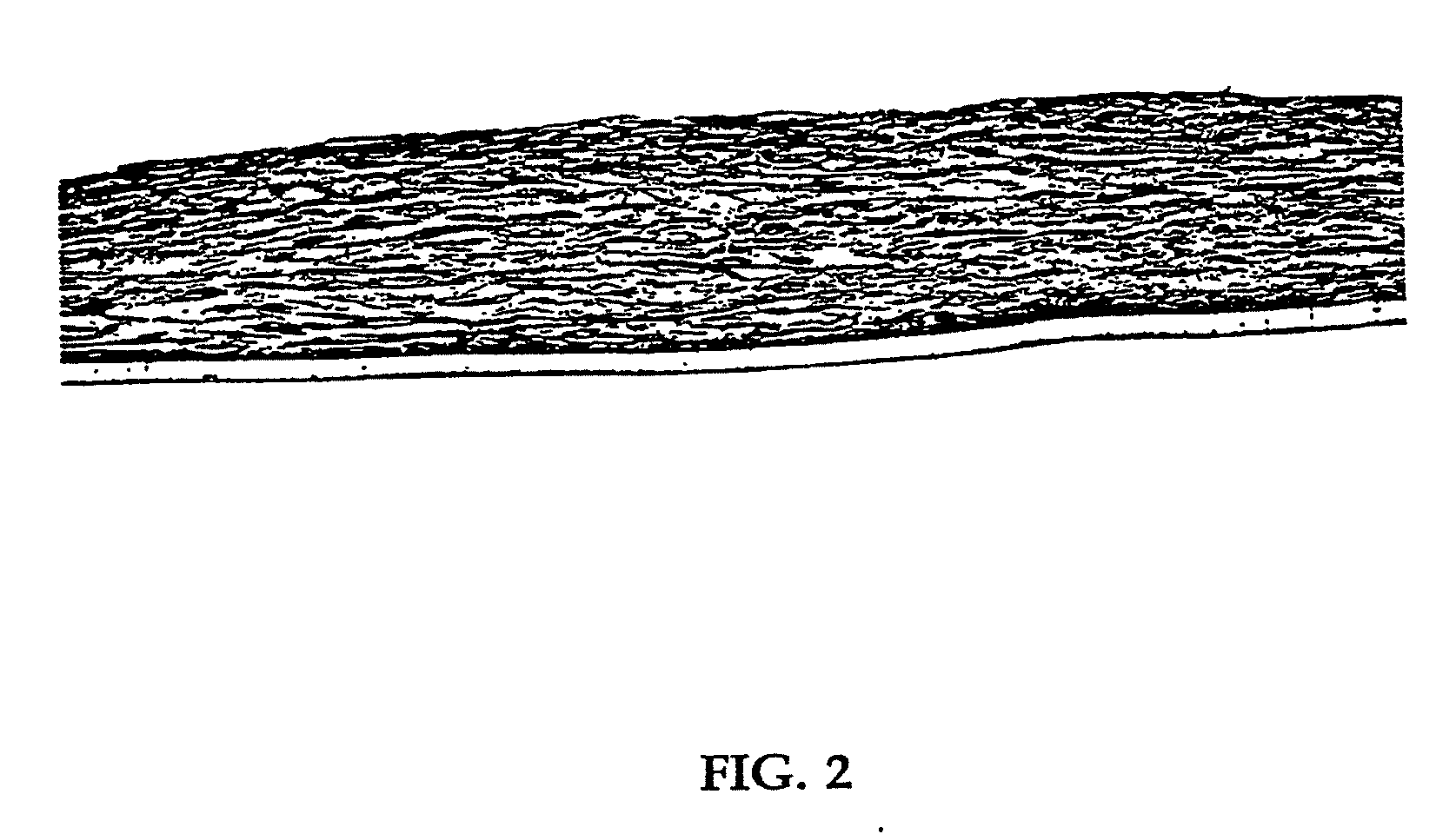
[0080] Human neonatal foreskin fibroblasts (originated at Organogenesis, Inc. Canton, Mass.) were seeded at 5×105 cells / 162 cm2 tissue culture treated flask (Costar Corp., Cambridge, Mass., cat #3150) and grown in growth medium. The growth medium consisted of Dulbecco's Modified Eagle's medium (DMEM) (high glucose formulation, without L-glutamine, BioWhittaker, Walkersville, Md.) supplemented with 10% newborn calf serum (NBCS) (HyClone Laboratories, Inc., Logan, Utah) and 4 mM L-glutamine (BioWhittaker, Walkersville, Md.). The cells were maintained in an incubator at 37±1° C. with an atmosphere of 10±1% CO2. The medium was replaced with freshly prepared medium every two to three days. After 8 days in culture, the cells had grown to confluence, that is, the cells had formed a packed monolayer along the bottom of the tissue culture flask, and the medium was aspirated from the culture flask. To rinse the monolayer...
example 2
Full Thickness Skin Construct
[0089] Using a dermal construct formed using the method described in Example 1, normal human neonatal foreskin epidermal keratinocytes (originated at Organogenesis, Inc. Canton, Mass.) were plated onto the cell-matrix construct to form the epidermal layer of the skin construct. The medium was aseptically removed from the culture insert and its surrounds. Normal human epidermal keratinocytes were scaled up to passage 4 from frozen subculture cell stock to confluence. Cells were then released from the culture dishes using trypsin-versene, pooled, centrifuged to form a cell pellet, resuspended in epidermalization medium, counted and seeded on top of the membrane at a density of 4.5×104 cells / cm2. The constructs are then incubated for 90 minutes at 37±1° C., 10% CO2 to allow the keratinocytes to attach. After the incubation, the constructs were submerged in epidermalization medium The epidermalization medium is composed of a 3:1 base mixture of Dulbecco's M...
example 3
In Vitro Formation of a Collagenous Matrix by Human Neonatal Foreskin Fibroblasts in Chemically Defined Medium
[0093] Human neonatal foreskin fibroblasts were expanded using the procedure described in Example 1. Cells were then resuspended to a concentration of 3×106 cells / ml, and seeded on to 0.4 micron pore size, 24 mm diameter tissue culture treated membrane inserts in a six-well tray at a density of 3.0×106 cells / TW (6.6×105 cells / cm2). These cells were then maintained as Example 1 with newborn calf serum omitted from the media throughout. More specifically the medium contained: a base 3:1 mixture of DMEM, Hams F12 medium (Quality Biologics, Gaithersburg, Md.), 4 mM GlutaMAX (Gibco BRL, Grand Island, N.Y.) and additives: 5 ng / ml human recombinant epidermal growth factor (Upstate Biotechnology, Lake Placid, N.Y.), 0.4 μg / ml hydrocortisone (Sigma, St. Louis, Mo.), 1×10−4 M ethanolamine (Fluka, Ronkonkoma, N.Y. cat. #02400 ACS grade), 1×10−4 M o-phosphoryl-ethanolamine (Sigma, St. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com