Eye-Drop Vaccine Containing Copolymer 1 for Therapeutic Immunization
a technology of eyedrops and copolymers, applied in the field of immunology, can solve the problems of no benefit to retinal ganglion cells suffering from iop elevated insult, damage to the nervous system, and no publication discloses immunizations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cop-1 Vaccination Protects RGCs From IOP-Induced Death When Given Without Vehicle
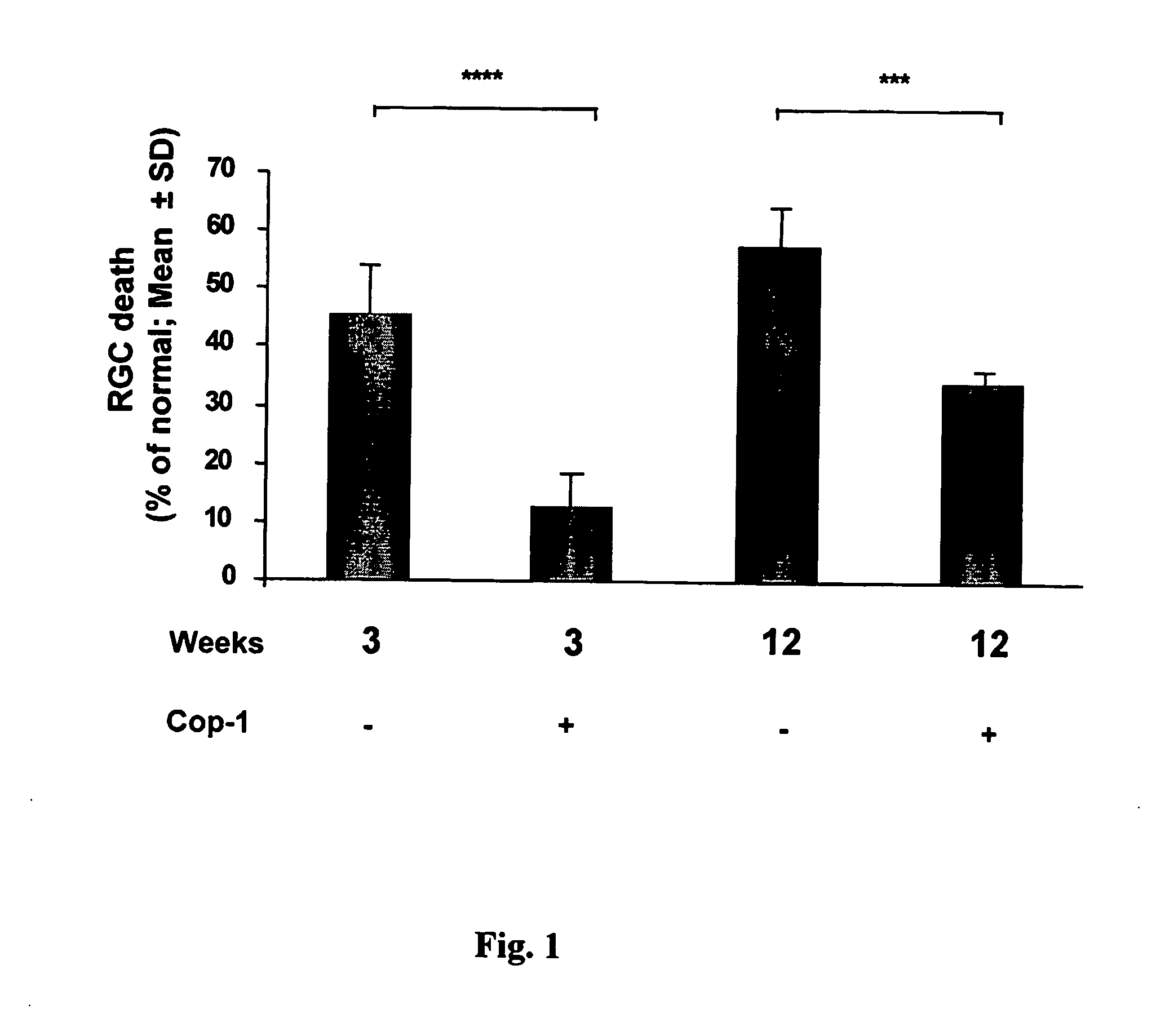
[0080] Previous studies have shown that Cop 1 emulsified in an adjuvant protects against IOP-induced RGC death. Here we examined whether the effect is long-lasting in a chronic model.
[0081] Animals were subjected to unilateral elevation of IOP and immunized on the day of laser treatment (to induce IOP elevation). Rats were subjected to chronic elevation of IOP on the day of the first laser irradiation. Animals were divided into 4 groups: two groups received Cop-1 emulsified in CFA and two groups received PBS in CFA. From one group of Cop-1-treated animals retinas were excised 3 weeks later and the second group received Cop-1 2, 6 and 9 weeks latter. From this group, retinas were excised 12 weeks after the first laser irradiation. Of the two PBS-CFA-treated groups, one was analyzed for RGC survival 3 weeks after the first laser irradiation, and one received additional injection of PBS-CFA at 2, 6 and 9...
example 2
Cop-1 Immunization Without Adjuvant
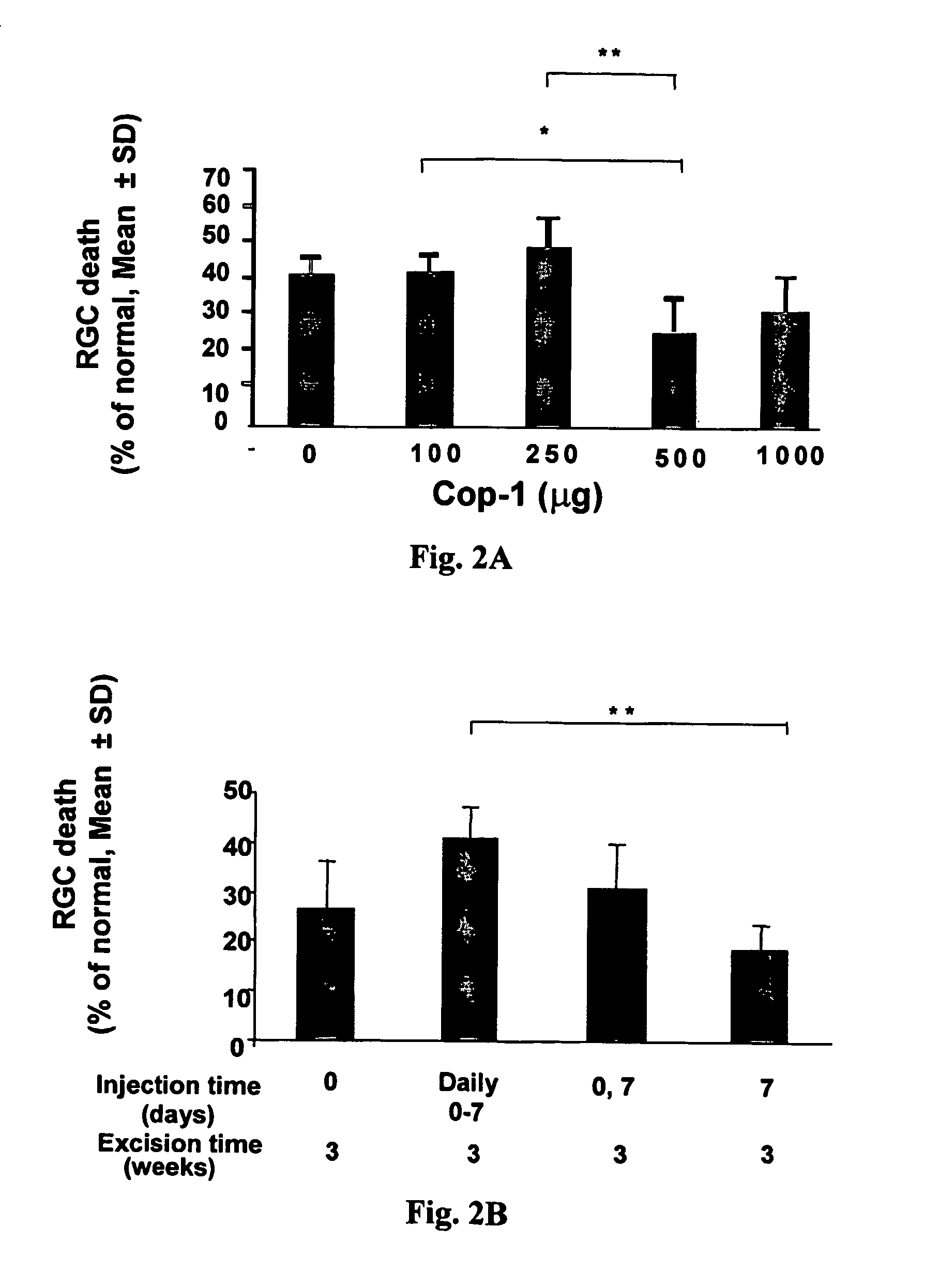
[0082] Since Cop-1 is a high molecular weight compound with multiple epitopes, we considered the possibility that it might be immunogenic even without adjuvant.
[0083] In a first experiment, rats were subjected to IOP elevation and received subcutaneous injection of different dosages of adjuvant-free Cop-1 at various dosages (100, 250, 500 and 1000 μg) on the first day of laser treatment; control animals received PBS. The results are depicted in FIG. 2A and show that the optimal effect could be achieved with 500 μg of Cop-1 injected subcutaneously to the rat; higher dosage or lower were less effective. The group treated with 500 μg showed the highest effect: 26.6±10% of RGC death as compared to 44±6% of RGC death in the group treated with 100 μg and 50.5±8% of RGC death in the group treated with 250 μg. In all groups 4-6 animals were included.
[0084] In another experiment, we also examined the timing and the frequency and found that injection on d...
example 3
Cop-1 Effect is T-Cell Dependent
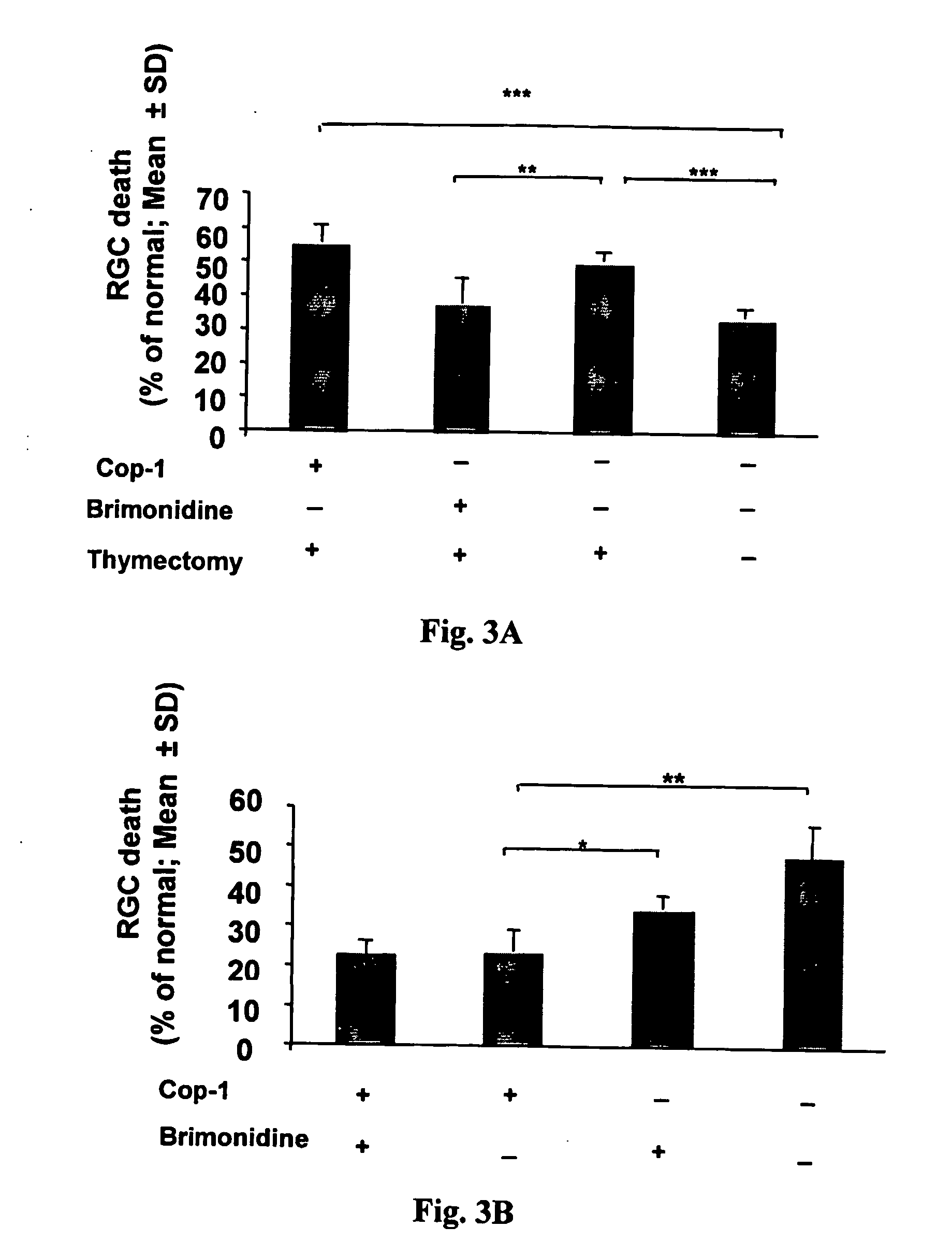
[0086] To verify that the effect of Cop-1 is indeed immune-mediated and does not act as a local drug, we administered Cop-1 to animals deprived of T cells in which IOP was elevated.
[0087] In a first experiment, normal adult rats and adult rats deprived of T cells (as a result of thymectomy at birth) were subjected to elevated IOP. Immediately after IOP elevation, animals received Cop-1, a single injection, or PBS, or daily brimonidine. Three weeks later retinae were excised and RGCs were counted. A lower percentage of RGCs died in non-thymectomized animals than in thymectomized animals following IOP elevation (34±3 vs. 50.2±3, p<0.001, n=6 and 5, respectively). As shown in FIGS. 3A and 3B, in the thymectomized animals treated with the α2-adrenoreceptor agonist glaucoma drug brimonidine, the loss of RGCs was lower than in the non-treated thymectomized or in the Cop-1-treated thymectomized rats [38±7 (n=6) vs. 55±5.2 (n=5); p=0.001]. The effect of bri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com