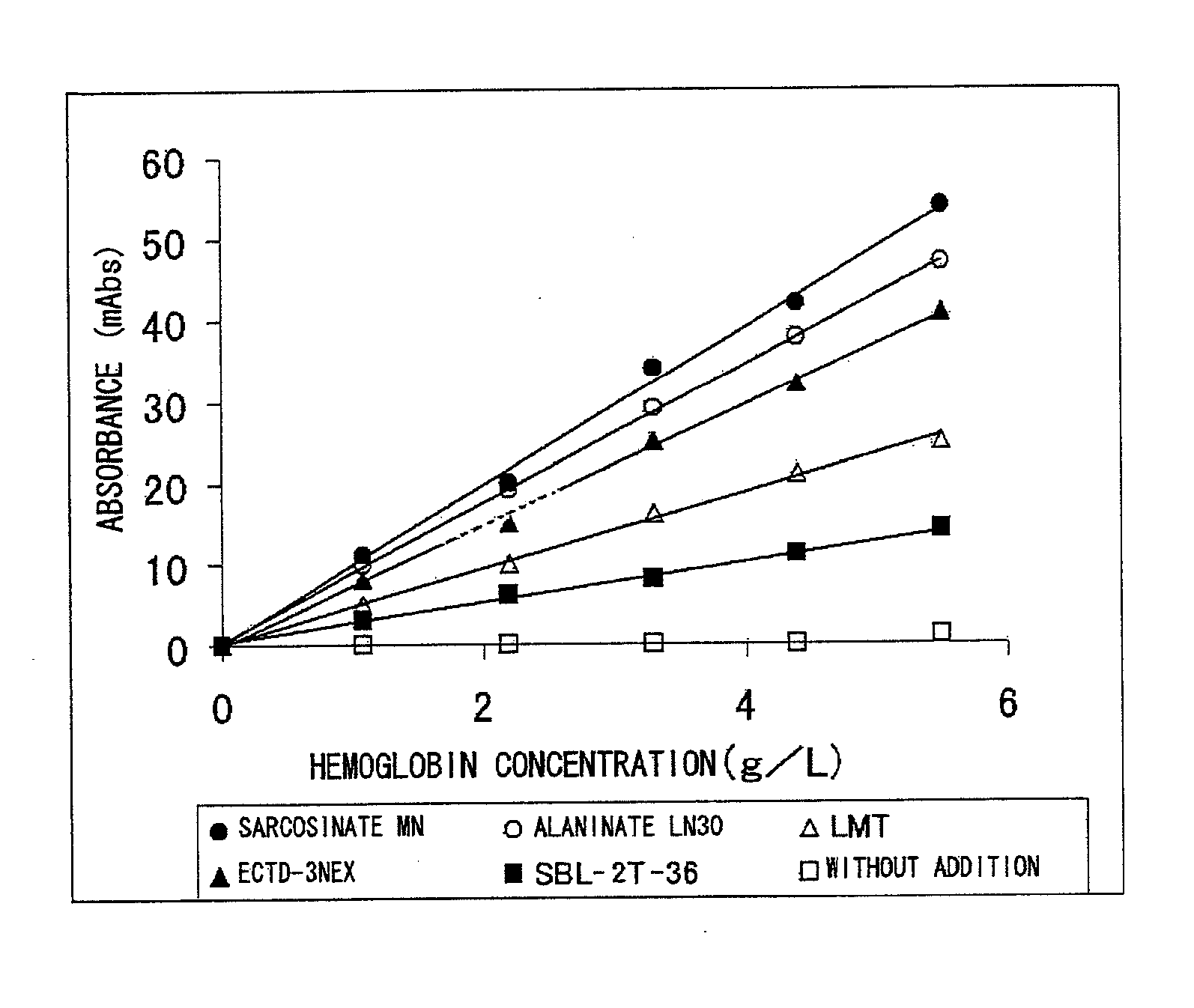
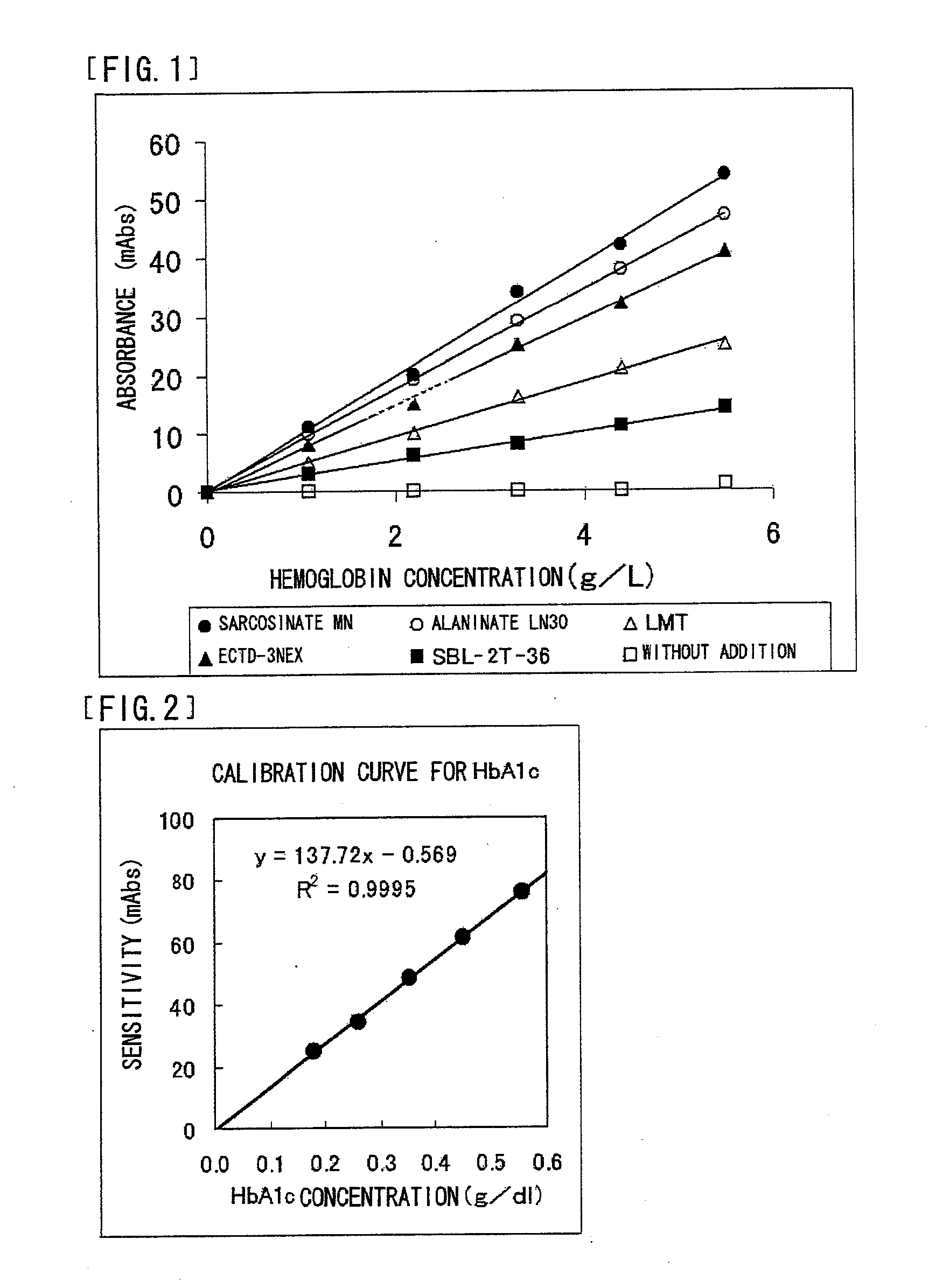
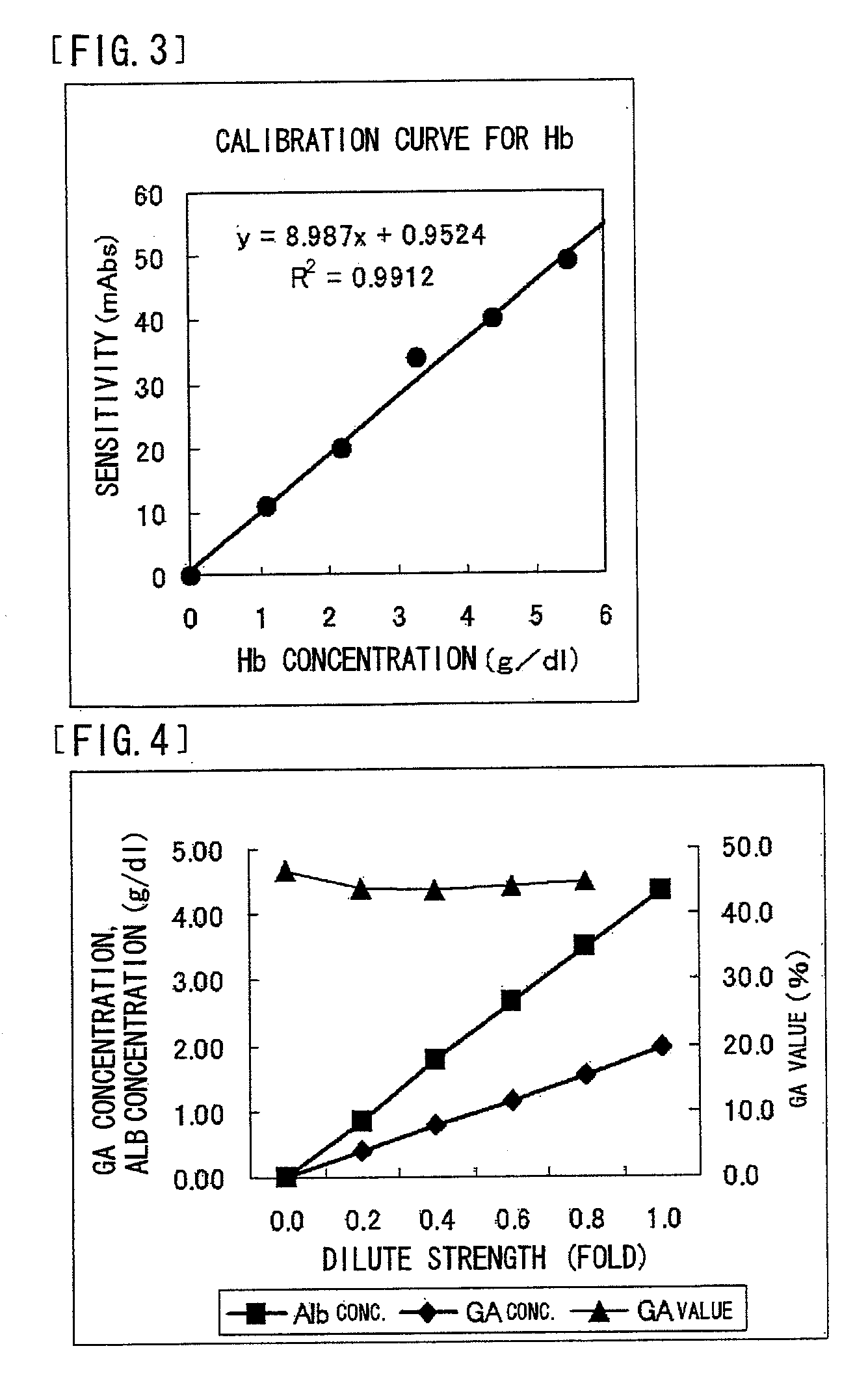
Reagent Containing Protease Reaction Promoter and/or Colorant Stabilizer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Screening of Protease Reaction Promoter
(Reagent)
[0181] 1) R-1: Reagent for Protein Decomposition
50 mMTris buffer solution (manufactured by Wako PureChemical Industries, Inc.), pH 7.04,000 U / mlNeutral protease (manufactured by Toyobo Co.,Ltd.)1.5 mlCaCl2 (manufactured by Wako Pure ChemicalIndustries, Inc.)+Test sample
[0182] 2) R-2: Reagent for Glycated Amino Acid Detection
50 mMTris buffer solution (manufactured by Wako PureChemical Industries, Inc.), pH 7.530 U / mlKAOD (derived from Curvularia clavata YH923;manufactured by Asahi Kasei Pharma Corporation, the methodof producing the same being described in JP-A-2004-275013)80 U / mlPeroxidase (manufactured by Sigma Aldrich Japan K.K.)80 μMDA-67 (manufactured by Wako Pure ChemicalIndustries, Inc.)
[0183] The following substances were independently used as a test sample. Note that concentrations described in parentheses each represent a final concentration of the substance in the reagent R1.
[0184] Tween-20 (1%), Brij35 (1%), Triton X...
example 2
Reagent Containing Protease Reaction Promoter
(Reagent)
[0191] Reagent for Hemoglobin Measurement
50 mMTris buffer solution (manufactured by Wako PureChemical Industries, Inc.), pH 7.0 1%Sarcosinate MN0.05%Sodium azide
[0192] Reagents as the above-mentioned reagent were also prepared separately except that AKYPO-RLM100, SMT, and SBL-2Z-36 were used, respectively, instead of sarcosinate MN.
example 3
Reagent Containing Protease Reaction Promoter and Protease
(Reagent)
[0193] 1) R-1: Reagent for Protein Decomposition
50 mMTris buffer solution (manufactured by Wako PureChemical Industries, Inc.), pH 7.04,000 U / mlNeutral protease (manufactured by Toyobo Co.,Ltd.; Toyozyme NEP)1.5 mlCaCl2 (Wako Pure Chemical Industries, Inc.) 1%Alaninate LN-300.05%Sodium azide
[0194] Specificity of the protease in this reagent was confirmed using glycated valyl-histidyl-leucyl-threonyl-proline and glycated valyl-leucyl-seryl-prolyl-alanine. It was found that the protease in this reagent has high specificity to the N terminal of a β chain of hemoglobin and does not substantially act on the N terminal of an α chain thereof. Further, similar substrate specificity was observed in cases of thermolysin and thermoase (manufactured by Daiwa Kasei K.K.) derived from Bacillus thermoproteolyticus Rokko, protease derived from Bacillus sp. ASP-842 FERM BP-08641, and protease derived from Lysobacter enzymogenes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com