Chemically modified G-CSF
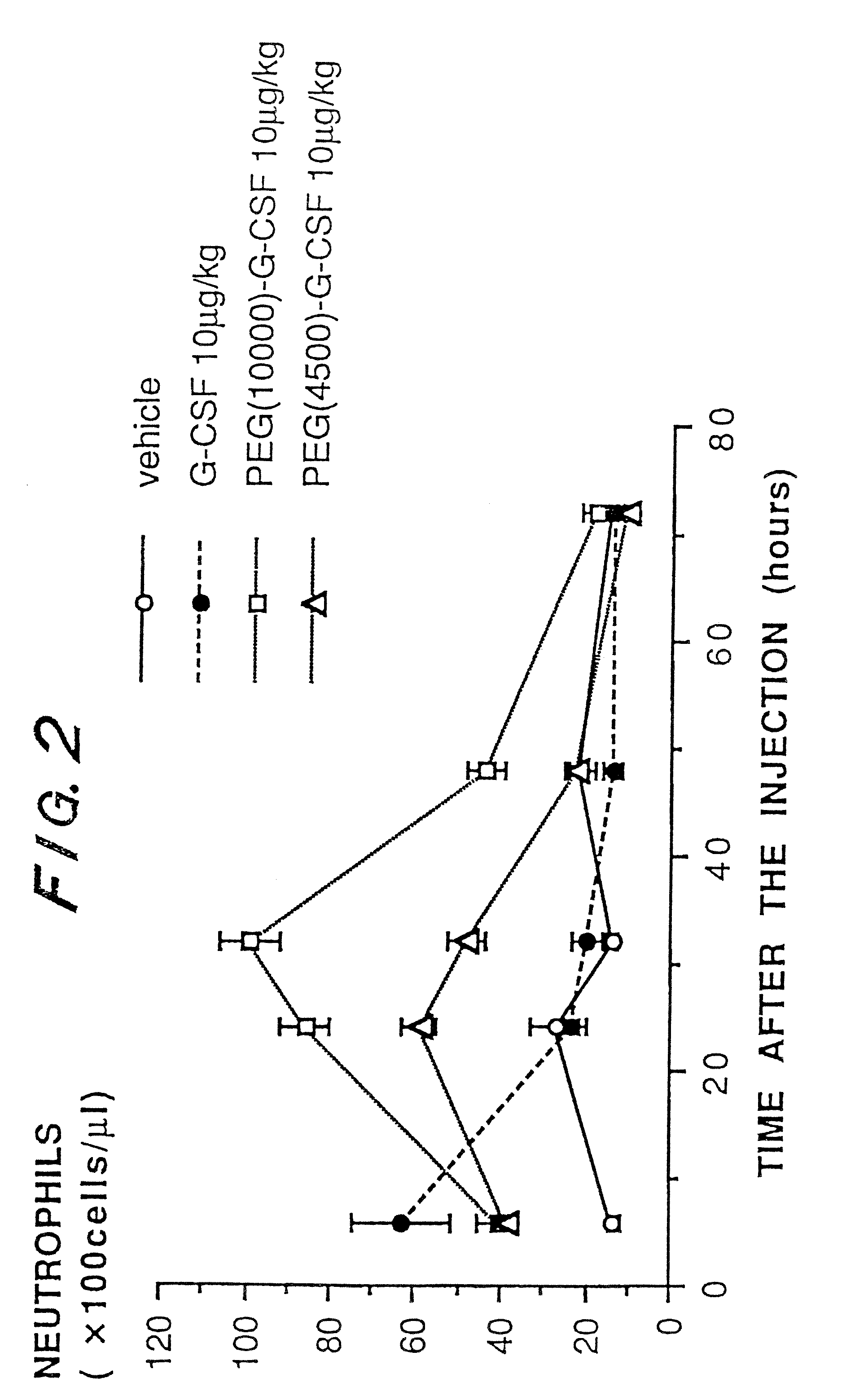
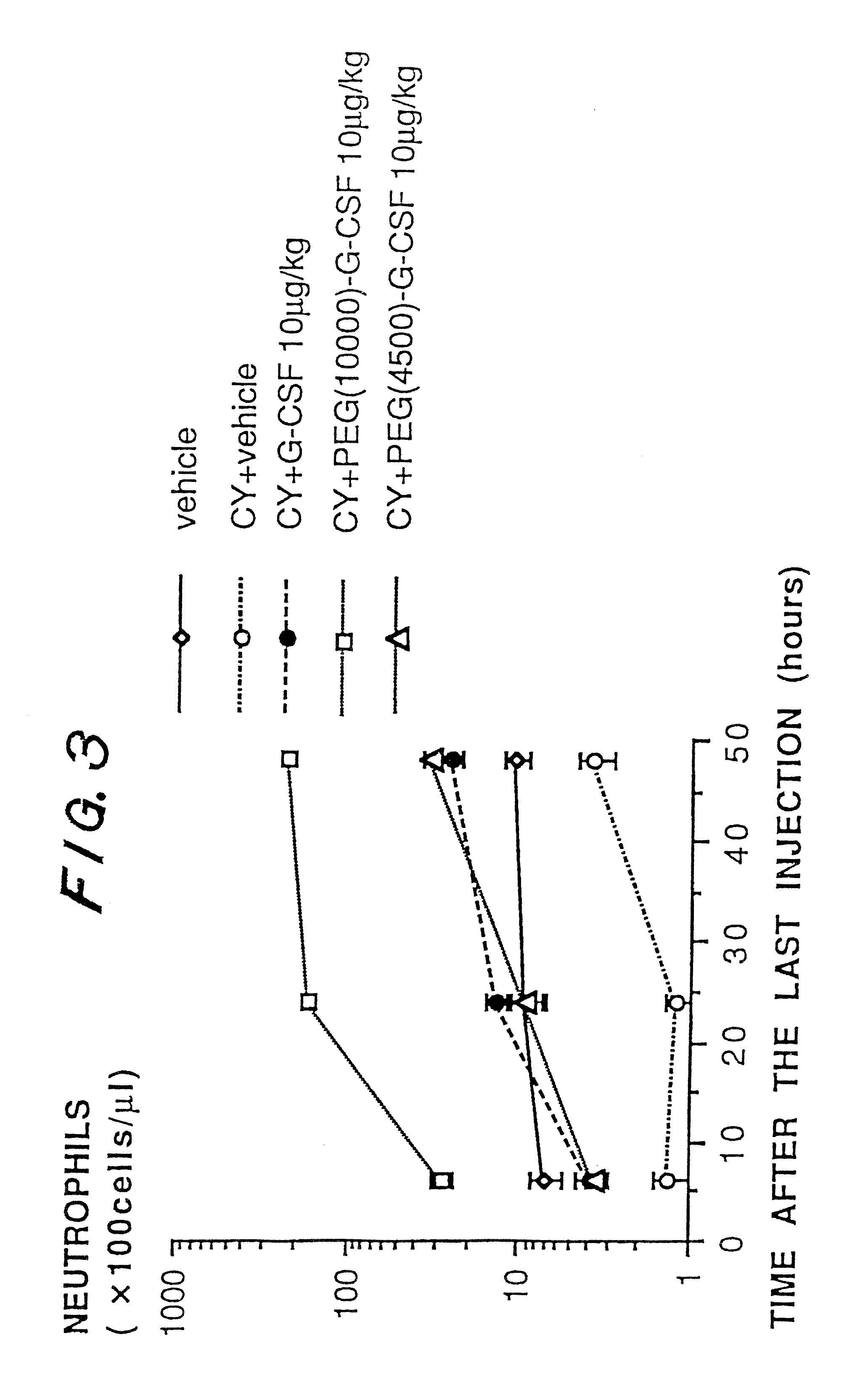
a technology of granulocyte colony and g-csf, which is applied in the direction of peptide/protein ingredients, immunoglobulins, peptides, etc., can solve the problems of reducing stability, publications have not disclosed an improvement in biological activity and pharmacokinetics, etc., to accelerate the recovery from neutropenia, prolong the pharmacological effect, and increase the number of neutrophils
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of PEG (4,500) G-CSF
[0031] Recombinant human G-CSF (Japanese Patent Application Laying Open KOHYO No. 500636 / 88) having the following amino acid sequence (SEQ ID NO: 2) was used for the chemical modification according to the present invention:
(SEQ ID NO: 2) MetThr Pro Leu Gly Pro Ala Ser Ser Leu Pro GlnSer Phe Leu Leu Lys Cys Leu Glu Gln Val ArgLys lle Gln Gly Asp Gly Ala Ala Leu Gln GluLys Leu Cys Ala Thr Tyr Lys Leu Cys His ProGlu Glu Leu Val Leu Leu Gly His Ser Leu GlyIle Pro Trp Ala Pro Leu Ser Ser Cys Pro SerGln Ala Leu Gln Leu Ala Gly Cys Leu Ser GlnLeu His Ser Gly Leu Phe Leu Tyr Gln Gly LeuLeu Gln Ala Leu Glu Gly Ile Ser Pro Glu LeuGly Pro Thr Leu Asp Thr Leu Gln Leu Asp ValAla Asp Phe Ala Thr Thr Ile Trp Gln Gln MetGlu Glu Leu Gly Met Ala Pro Ala Leu Gln ProThr Gln Gly Ala Met Pro Ala Phe Ala Ser AlaPhe Gln Arg Arg Ala Gly Gly Val Leu Val AlaSer His Leu Gln Ser Phe Leu Glu Val Ser TyrArg Val Leu Arg His Leu Ala Gln Pro
[...
example 2
Characterization of PEG (4,500) G-CSF
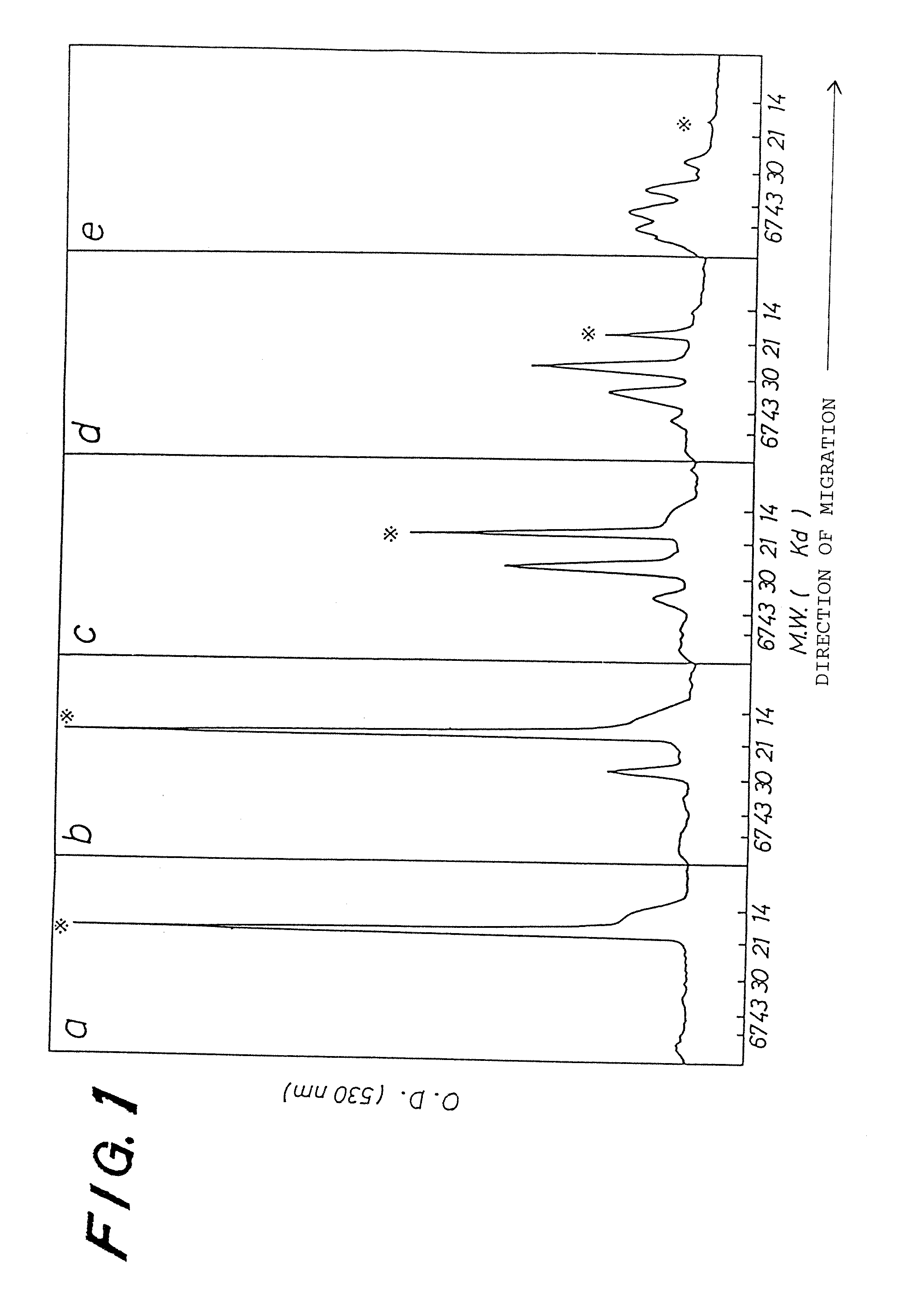
[0034] PEG (4,500) G-CSF prepared in EXAMPLE 1 was characterized by the number of unmodified amino groups and a molecular weight estimated by SDS-PAGE.
[0035] The number of the unmodified amino groups was determined by reacting them with 0.1% TNBS in 4% NaHCO.sub.3 followed by measurement of absorbance at 335 nm (Habeeb et al., Anal. Biochem., 14, pp. 328-336, (1966)).
[0036] The molecular weight of PEG (4,500) G-CSF was determined by SDS-PAGE (16% gel, CBB staining) according to a method of Laemli, Nature, 227, p. 680, 1970. Each lane on the gel was scanned by using a chromato-scanner (SHIMADZU CORPORATION: CS-930) after staining.
[0037] When a molar ratio of the activated PEG to the number of free amino groups of human G-CSF increased, the extent of the modification also increased. The product prepared in said molar ratio of 1 has in addition to a band corresponding to an intact human G-CSF (19K) another band with an apparent molecular weight...
example 3
Preparation of PEG (10,000) G-CSF
[0040] The same human G-CSF as used in EXAMPLE 1 was modified by an activated polyethylene glycol (an activated PEG 2; Seikagaku Kogyo K.K.) with a molecular weight of about 10,000 having the following formula:
which had been prepared by reacting polyethylene glycol monomethyl ether with cyanuric chloride.
[0041] The human G-CSF was incubated with the activated PEG 2 at 5 times the molar amount of free amino groups of the human G-CSF in 0.25M sodium borate buffer solution (pH 10.0) for 1 hr at room temperature. The resulting product was applied to Sephadex G25 which had been equilibrated with 10 mM NH4 HCO3 for buffer-exchange, and then to DEAE ion-exchange chromatography to separate the PEG-modified human G-CSF from an unreacted human G-CSF and reagent. The estimation of a molecular weight of the product by SDS-PAGE as in EXAMPLE 2 has revealed that its average molecular weight is about 45K with distributed among 30K (10%), 40K (70%) and 66K (20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com