Histone deacetylase inhibitors as therapeutics for neurological diseases
a technology of histone deacetylase and inhibitors, which is applied in the field of histone deacetylase inhibitors, can solve problems such as treating disease symptoms, and achieve the effects of restoring the normal function of a gene, restoring the transcription of frataxin mrna, and restoring the normal function of frataxin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials & Methods
[0131] Cell Culture
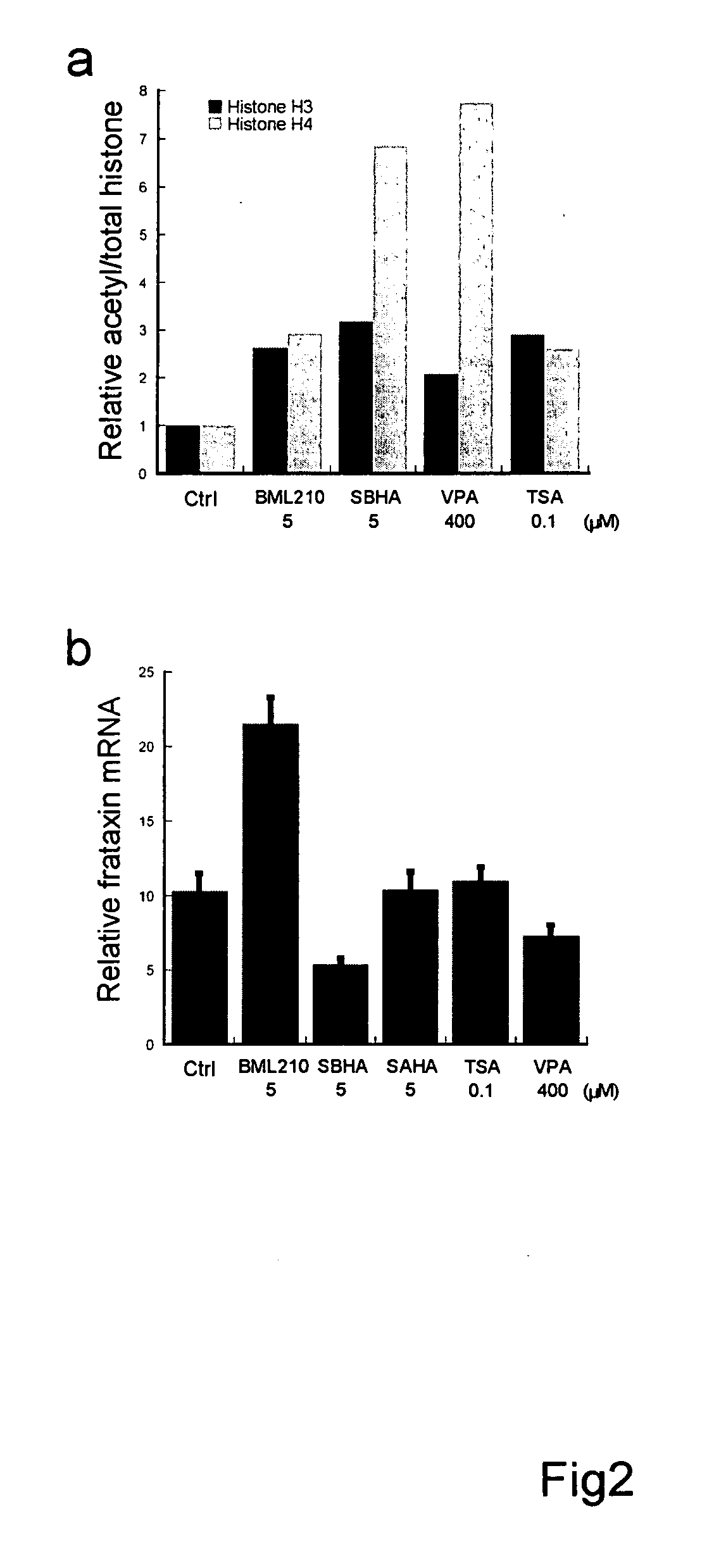
[0132] Epstein Barr virus transformed lymphoblast cell lines GM15850 from a FRDA patient (alleles with 650 and 1030 GAA repeats in the frataxin gene, from the Coriell Cell Repository, Camden, N.J.), and GM15851 from an unaffected sibling (normal range of repeats), were propagated in RPMI 1640 medium with 2 mM L-glutamine and 15% fetal bovine serum at 37° C. in 5% CO2. Cell growth and morphology were monitored by phase contrast microscopy, and viability by trypan blue exclusion. HDAC inhibitors were dissolved in DMSO and added to the culture medium at the concentrations indicated in the table and figure captions, for the indicated times. The final DMSO concentration in the culture medium did not exceed 0.5% (v / v). All control samples were treated with the same concentration of DMSO lacking compounds. The suppliers of the HDAC inhibitors were: valproic acid (VPA), Calbiochem (San Diego, Calif.); trichostatin A (TSA), suberoyl bis-hydroxamic acid...
example 2
Synthesis of Bis-Amides HDAC Inhibitors by a Novel Two-step Procedure
[0143] General Synthetic Procedure
[0144] Adipic acid 1a (n=3, Scheme 1), pimelic acid 1b (n=4) or suberic acid 1c (n=5) were used as the starting materials for the synthesis of the HDAC inhibitors. The synthetic scheme is as shown below.
[0145] By reaction with acetic anhydride under reflux, the dicarboxylic acids undergo intramolecular ring closure to compounds 2a, 2b and 2c. In contrast to published results (Wong et al., J. Am. Chem. Soc. 125:5586-7 (2003)), these anhydrides are further reacted without purification under ring opening conditions with aniline to the precursor compounds 3a, 3b and 3c in about 90% yield. Potent coupling conditions with 1-(3-dimethylamino-propyl)-3-ethylcarbodiimide hydrochloride (EDC) and 1-hydroxy-7-azabenzotriazole (HOAt) produce a high conversion rate, resulting in fast reactions with high yields. By using these conditions the yield of 4b increased to 64% (compared to 33% (Wong...
example 3
Histone Compositions of Active & Repressed Frataxin Alleles
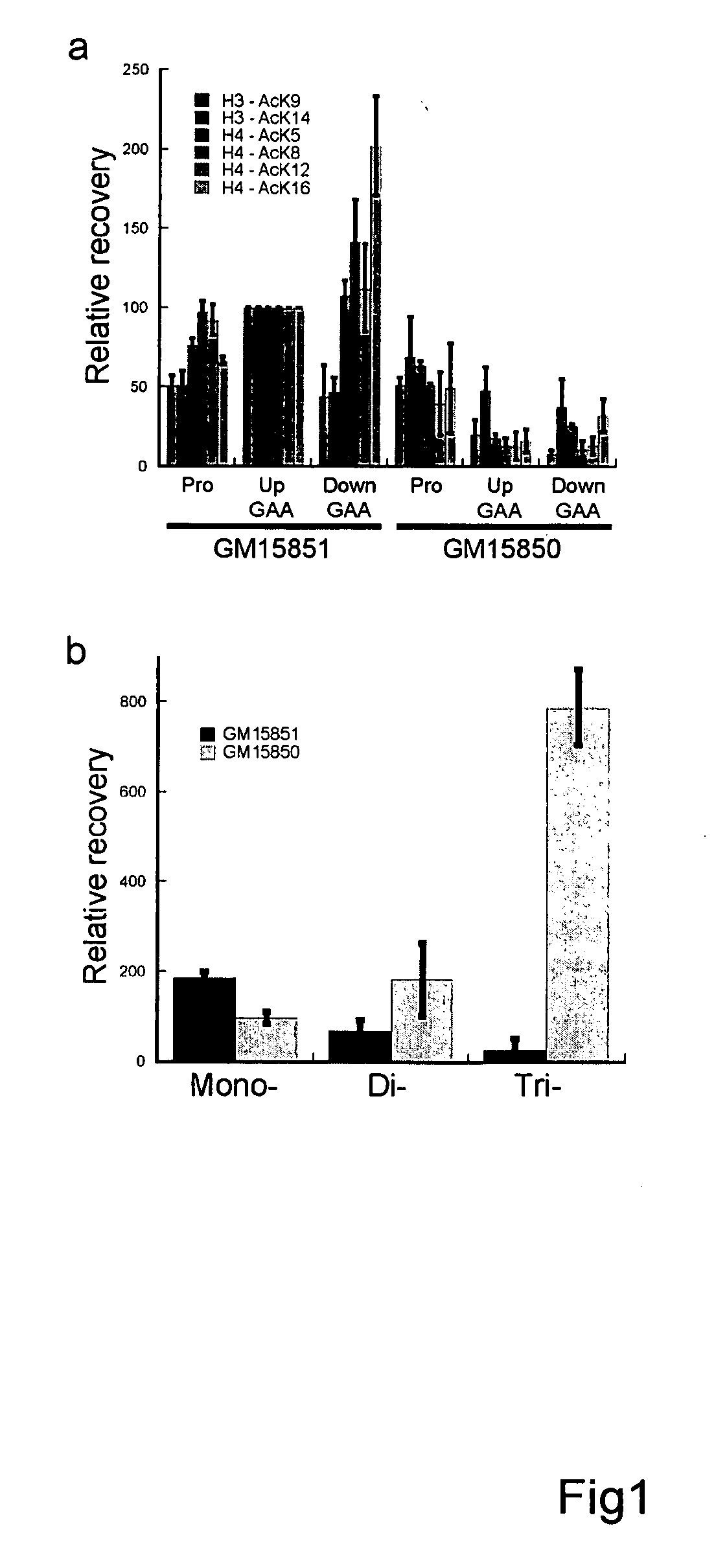
[0224] To assess whether histone modifications play a role in gene silencing in FRDA, the histone acetylation state of the frataxin gene in an Epstein Barr virus transformed lymphoid cell line derived from an FRDA patient (line GM15850, alleles with 650 and 1030 GAA·TTC repeats in the frataxin gene, from the NIGMS Human Genetic Cell Repository, Coriell Institute, Camden, N.J.) was monitored by chromatin immunoprecipitation (ChIP) with antibodies to the acetylated forms of histones H3 and H4. For comparison, we used a similar cell line from a normal sibling of this patient (line GM15851, normal range of repeats). As expected, the cell line from the FRDA patient has a markedly lower level (13±6%, range of 20 determinations (Burnett et al. P.N.A.S. 103: 11497-502 (2006)) of frataxin mRNA compared to the cell line from the unaffected sibling, as determined by quantitative real time / reverse transcriptase PCR (qRT-PCR, see below)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical inductance | aaaaa | aaaaa |
| Absorption cross section | aaaaa | aaaaa |
| Electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com