Enantioselective oligomerization of alpha-hydroxy carboxylic acids and alpha-amino acids
a technology which is applied in the field of enantioselective oligomerization of alpha-hydroxy carboxylic acids and alpha-amino acids, can solve the problems of not being able to efficiently utilize feed, unable to provide protein or amino acids to animals for absorption, and not being able to protect animals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Methionine Oligomers and HMB-Methionine Co-Oligomers
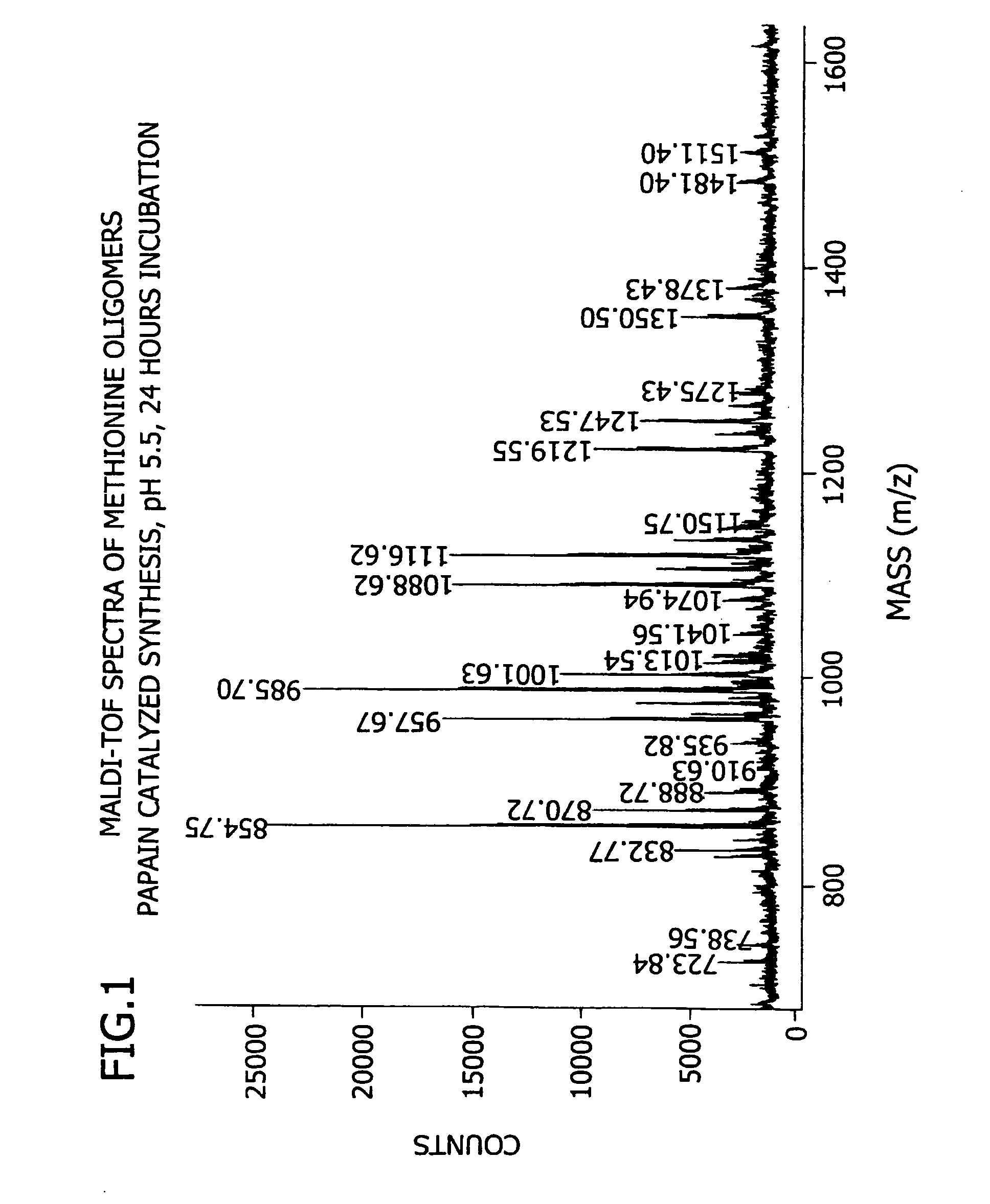
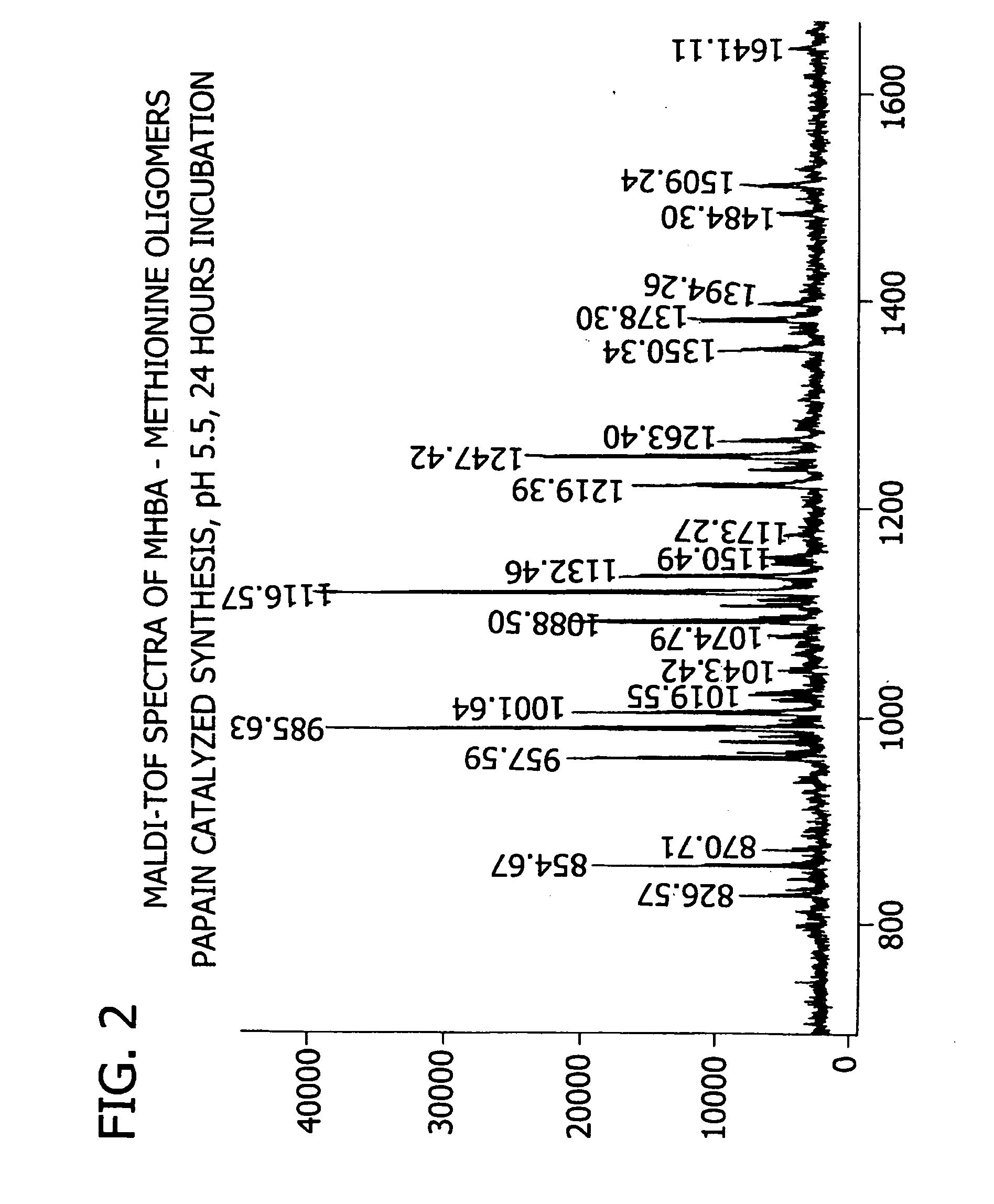
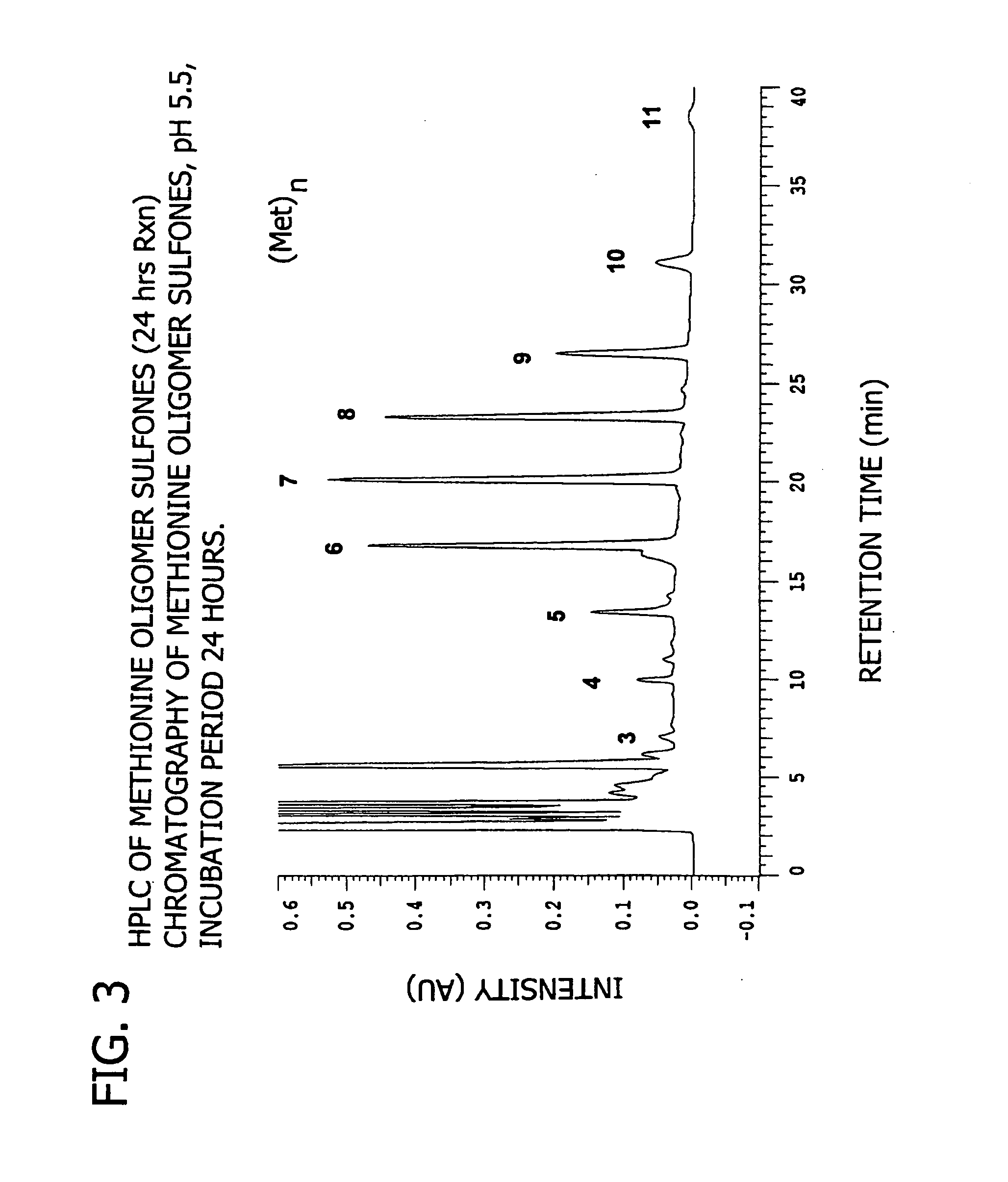
[0180] This example demonstrates the enzymatic synthesis of oligomers comprising methionine and co-oligomers comprising HMB-methionine, as well as their characterization using reverse-phase HPLC and matrix assisted laser desorption ionization-time of flight mass spectroscopy (MALDI-TOF MS) analysis.
Co-Oligomerization
[0181] In a first synthesis, the experiment was generally conducted in accordance with reaction conditions for the papain-catalyzed oligomerization of methionine analogs as described by Arai et al. in Agric. Biol. Chem., 43(5), 1069-1074 (1979). The oligomerization comprised forming a reaction mixture at a temperature of 37° C. consisting of nanopure filtered water (10 mL) containing HMB ethyl ester (0.7 M) and methionine ethyl ester (0.7 M) along with L-cysteine (0.1 M), EDTA (10 mM), sodium citrate (1M) and 1% papain (by weight of the monomer) at a pH of 5.5. The mixture was allowed to incubate for 24...
example 2
Oligomerization and Co-Oligomerization of Lysine and HMB
[0195] This example demonstrates four alternative procedures for the enzymatic synthesis of oligomers comprising lysine and co-oligomers comprising HMB-lysine. The experiment was designed to compare three novel synthesis procedures to that of Puigserver et. al.1 who reported a procedure for papain catalyzed polymerization of lysine.
[0196] In general, protease-catalyzed synthesis of water insoluble amino acid oligomers in aqueous media is driven by precipitation. The synthesis of water soluble oligomers of amino acids, such as lysine can be controlled only in mixed phase systems where the equilibria is shifted in favor of the synthesis of polypeptides due to enhanced partitioning of peptide in the organic phase. Puigserver et. al. reported a procedure for papain catalyzed polymerization of lysine which involved the binding of papain to modified PEG (MW 2000 or 5000). The bound enzyme was then used to synthesize poly lysine in ...
example 3
[0201] HMB-methionine and HMB-lysine co-polymers were synthesized enzymatically through a papain-catalyzed reaction along with poly-methionine and poly-lysine (as controls) as described in Examples 1 and 2. The biological release of the amino acids from the oligomers was examined using several digestive enzymes including pepsin, trypsin, chymotrypsin, intestinal peptidase and carboxypeptidase. The oligomers were dissolved at 10 mg / mL in 0.15 HCl (pH 2.5) or 50 mM KPO4 (pH 7.5). Samples (0.5 mL) were incubated with 10 units of each enzyme for 2 hours at 37° C. The extent of digestion was quantified by measurement of newly released amino groups and their reaction with o-Phthalaldehyde (OPA) and 2,4,6-trinitrobenzene sulfonic acid (TNBSA). Acid hydrolysis was prepared by complete hydrolysis of 10 mg / mL polymers in 6 M HCl for 24 hours at 110° C. Results are summarized below in Table 3.
[0202] Results show that HMB-methionine and HMB-lysine can be hydrolyzed by strong acid and heat. HMB...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| particle sizes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com