A device for enhanced epithelial permeation of y2 receptor-binding peptides
a technology of y2 receptor and epithelial permeation, which is applied in the field of enhanced epithelial permeation of y2 receptor-binding peptides, can solve the problems of reducing life span, affecting the quality of life, so as to prevent or cure diabetes, promote weight loss in an individual, and induce satiety in an individual.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0310] An exemplary formulation for enhanced nasal mucosal delivery of peptide YY following the teachings of the instant specification was prepared and evaluated as follows:
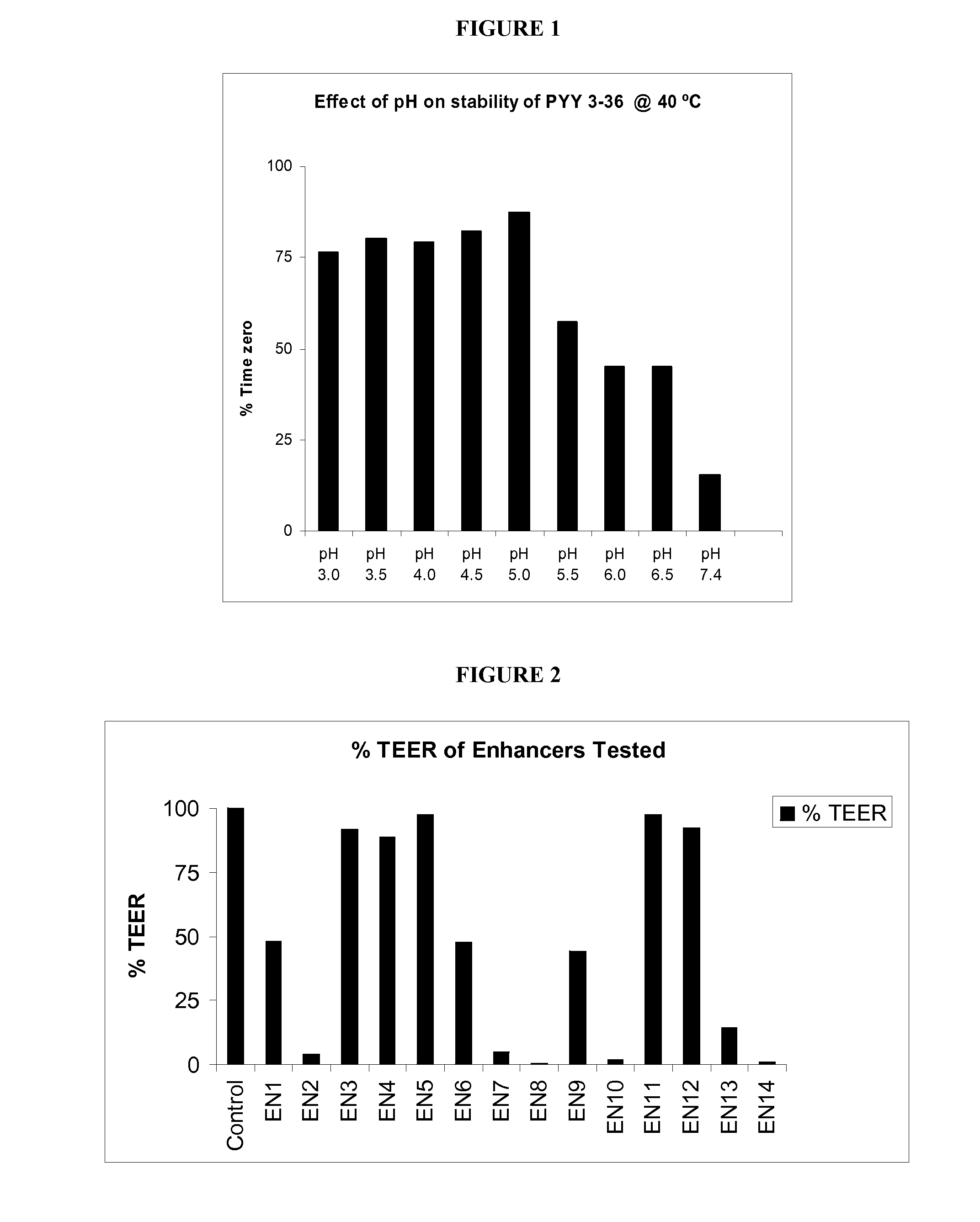
TABLE 1Peptide YY Formulation CompositionPeptide YY3-36Formu-Per 100 mllationsSampleMucosal Delivery Enhancing AgentA60 μgPhosphate-buffered saline (0.8%)pH 7.4 (Control 1)B60 μgPhosphate-buffered saline (0.8%)pH 5.0 (Control 2)C60 μgL-Arginine (10% w / v)D60 μgPoly-L-Arginine (0.5% w / v)E60 μgGamma-Cyclodextrin (1% w / v)F60 μgα-Cyclodextrin (5% w / v)G60 μgMethyl-β-Cyclodextrin (3% w / v)H60 μgn-Capric Acid Sodium (0.075% w / v)I60 μgChitosan (0.5% w / v)J60 μgL-α-phosphatidylcholine didecanyl(3.5% w / v)K60 μgS-Nitroso-N-Acetyl-Penicillamine(0.5% w / v)L60 μgPalmotoyl-DL-Carnitine (0.02% w / v)M60 μgPluronic-127 (0.3% w / v)N60 μgSodium Nitroprusside (0.3% w / v)O60 μgSodium Glycocholate (1% w / v)P60 μgF1: Gelatin, DDPC, MBCD, EDTAF 1L-α-phosphatidylcholine didecanyl (0.5%w / v) Methyl β Cyclodextrin (3% w / v)EDTA (0.1% w / v, Inf. Conc...
example 2
Nasal Mucosal Delivery—Permeation Kinetics And Cytotoxicity
[0311] 1. Organotypic Model
[0312] The following methods are generally useful for evaluating nasal mucosal delivery parameters, kinetics and side effects for peptide YY within the formulations and method of the invention, as well as for determining the efficacy and characteristics of the various intranasal delivery-enhancing agents disclosed herein for combinatorial formulation or coordinate administration with peptide YY.
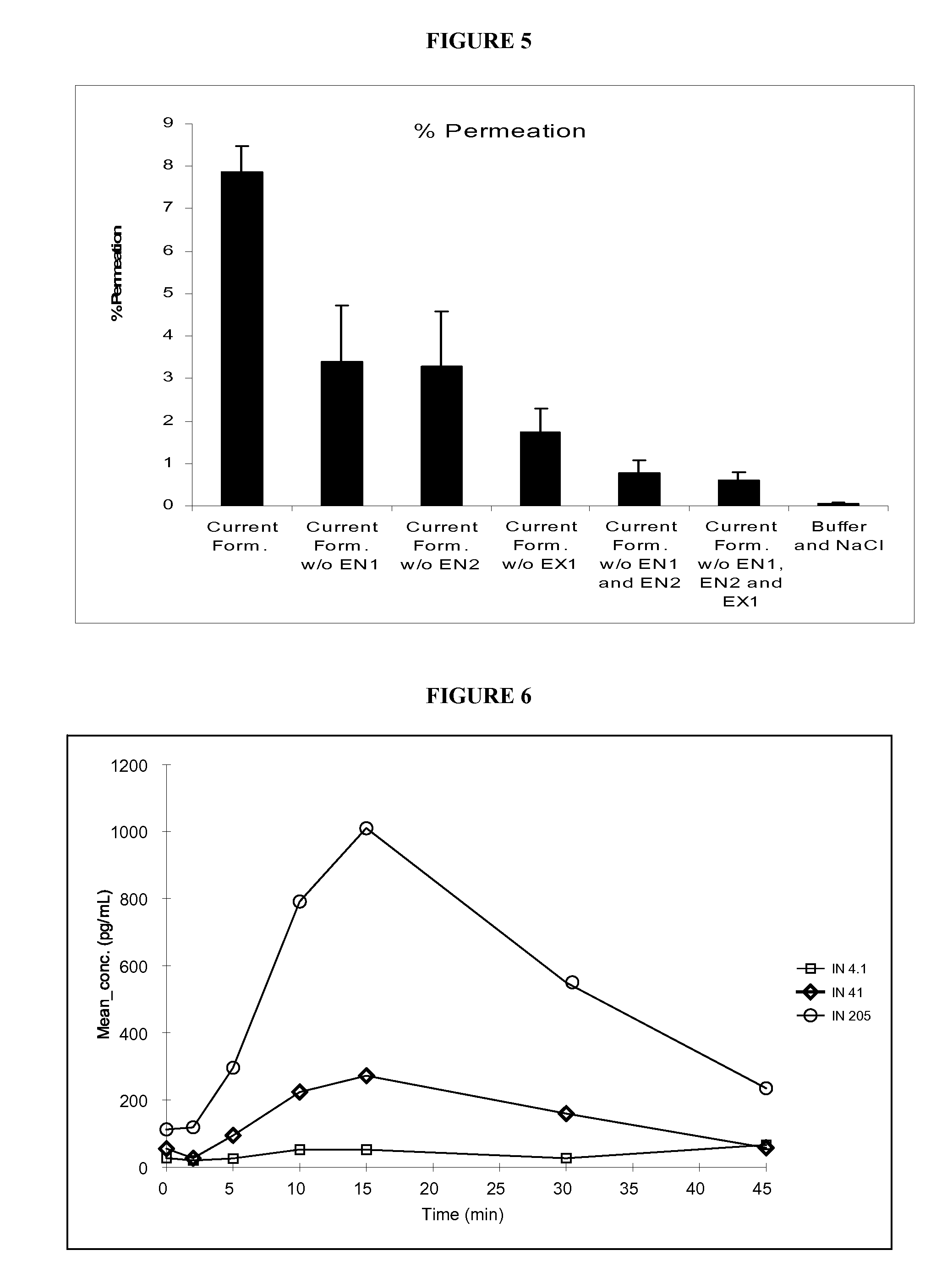
[0313] Permeation kinetics and cytotoxicity are also useful for determining the efficacy and characteristics of the various mucosal delivery-enhancing agents disclosed herein for combinatorial formulation or coordinate administration with mucosal delivery-enhancing agents. In one exemplary protocol, permeation kinetics and lack of unacceptable cytotoxicity are demonstrated for an intranasal delivery-enhancing agent as disclosed above in combination with a biologically active therapeutic agent, exemplified...
example 3
Formulation P (Peptide YY) of the Present Invention in Combination with Triamcinolone Acetonide Corticosteroid Improves Cell Viability
[0372] The present example provides an in vitro study to determine the permeability and reduction in epithelial mucosal inflammation of an intranasally administered peptide YY, for example, human peptide YY, in combination with a steroid composition, for example, triamcinolone acetonide, and further in combination with one or more intranasal delivery-enhancing agents. The study involves determination of epithelial cell permeability by TER assay and reduction in epithelial mucosal inflammation as measured by cell viability in an MTT assay by application of an embodiment comprising peptide YY and triamcinolone acetonide.
[0373] Formulation P (see Table 1 above) is combined in a formulation with triamcinolone acetonide at a dosage of 0.5, 2.0, 5.0, or 50 μg. Normal dose of triamcinolone acetonide, (Nasacort®, Aventis Pharmaceuticals) for seasonal allerg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com