Method of inhibiting metal corrosion
a metal corrosion and metal technology, applied in the direction of solid-state diffusion coating, coating, metallic material coating process, etc., can solve the problems of adversely affecting product quality, toxic chemical agents used to inhibit corrosion, metal materials are corroded, etc., to reduce the tendency of metal corrosion, reduce the corrosivity of reaction solutions, and improve the effect of oxidizability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
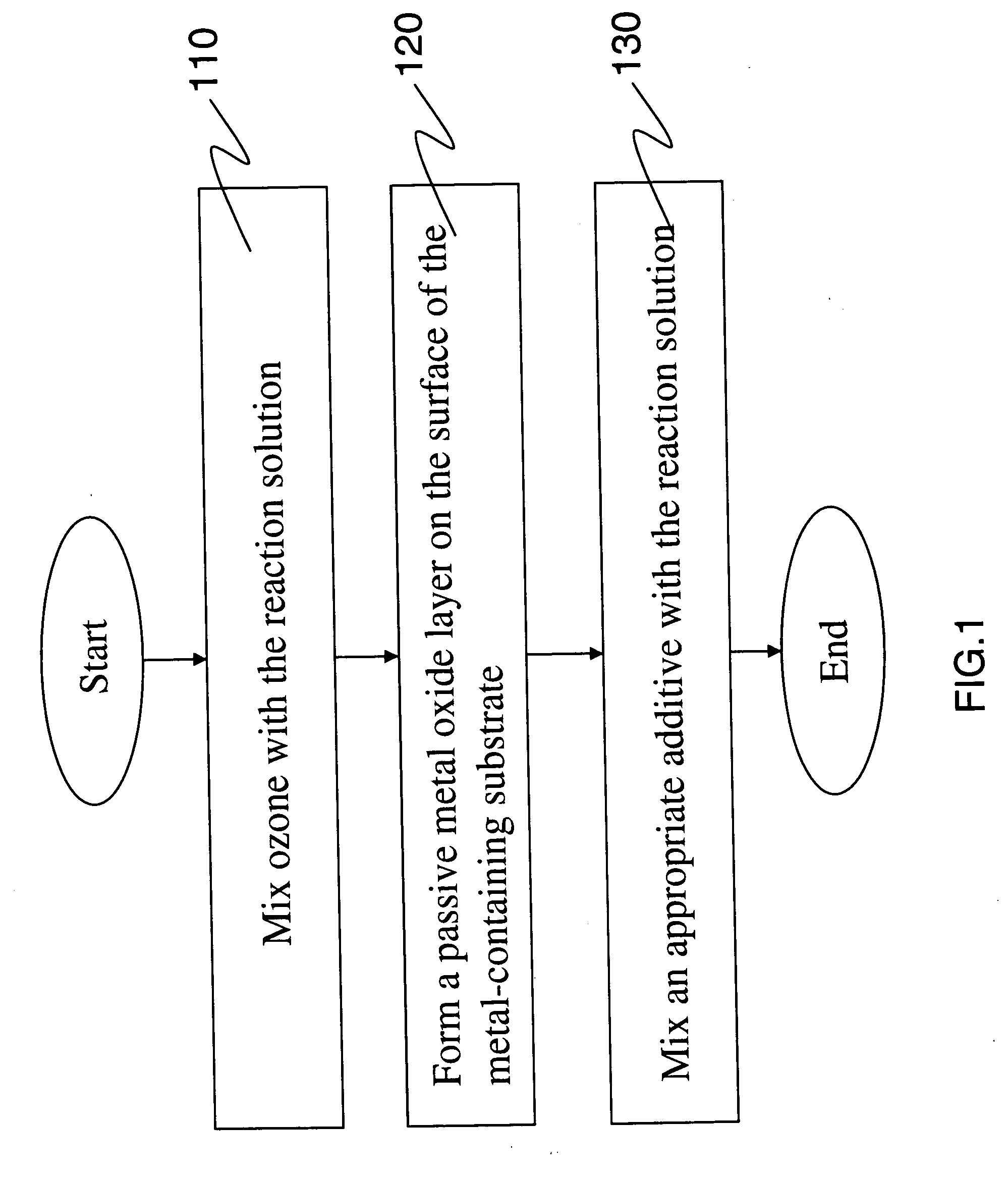
[0013] The method of inhibiting metal corrosion of the present invention is applicable to metal-containing substrate having a surface in contact with a reaction solution. The metal-containing substrate surface may be caused to contact with the reaction solution in different ways, such as immersing the metal-containing substrate in the reaction solution, or spraying the reaction solution on the metal-containing substrate. Please refer to FIG. 1 that is a flowchart showing the steps included in the method of inhibiting metal corrosion according to a preferred embodiment of the present invention. As shown, in a first step (110), ozone is mixed with the reaction solution. In a second step (120), a passive metal oxide layer is formed on the surface of the metal-containing substrate. And, in a third step (130), an adequate amount of additive is mixed with the reaction solution. In the step of mixing ozone with the reaction solution, either a solution containing ozone is added into the rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com