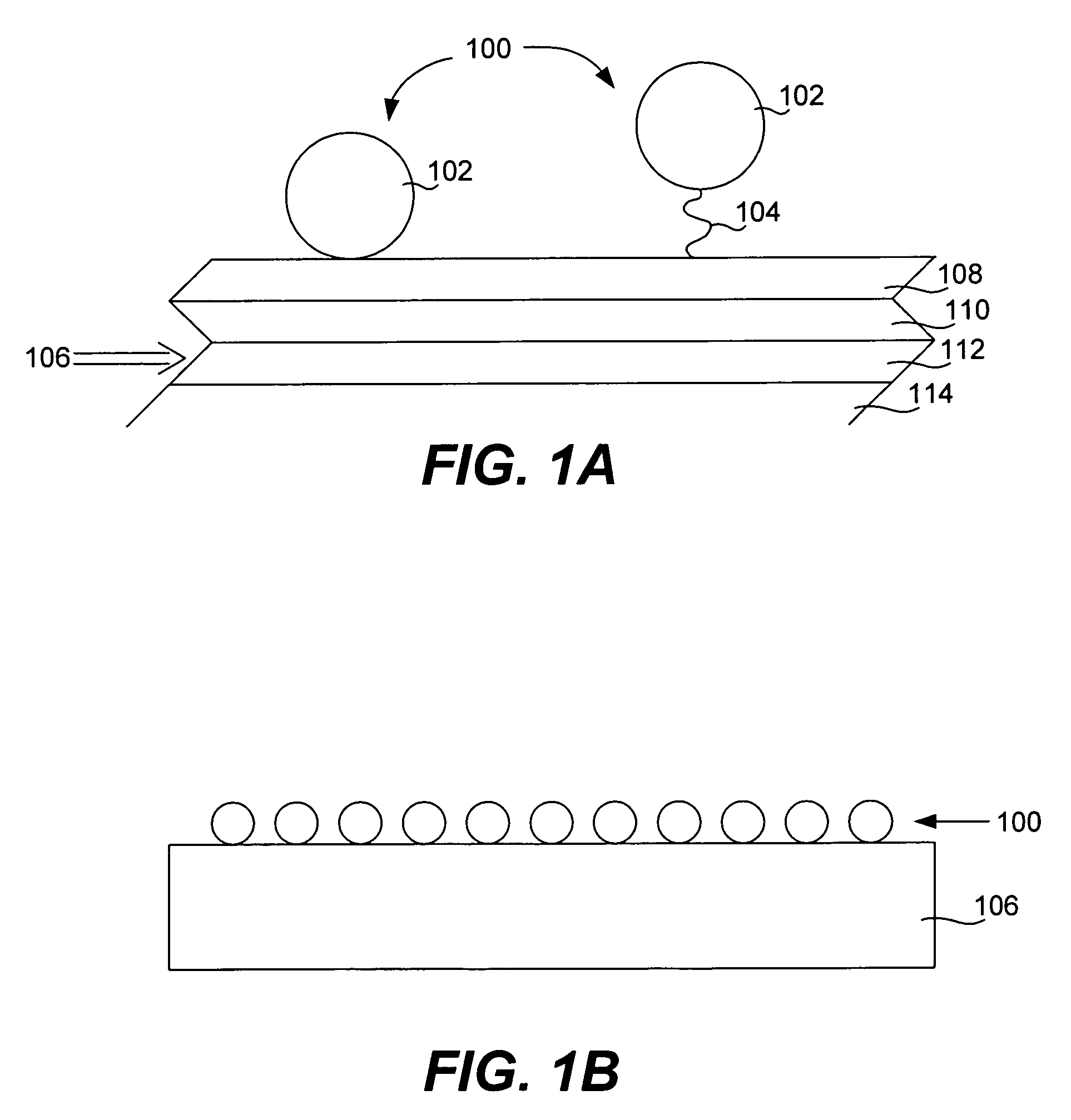
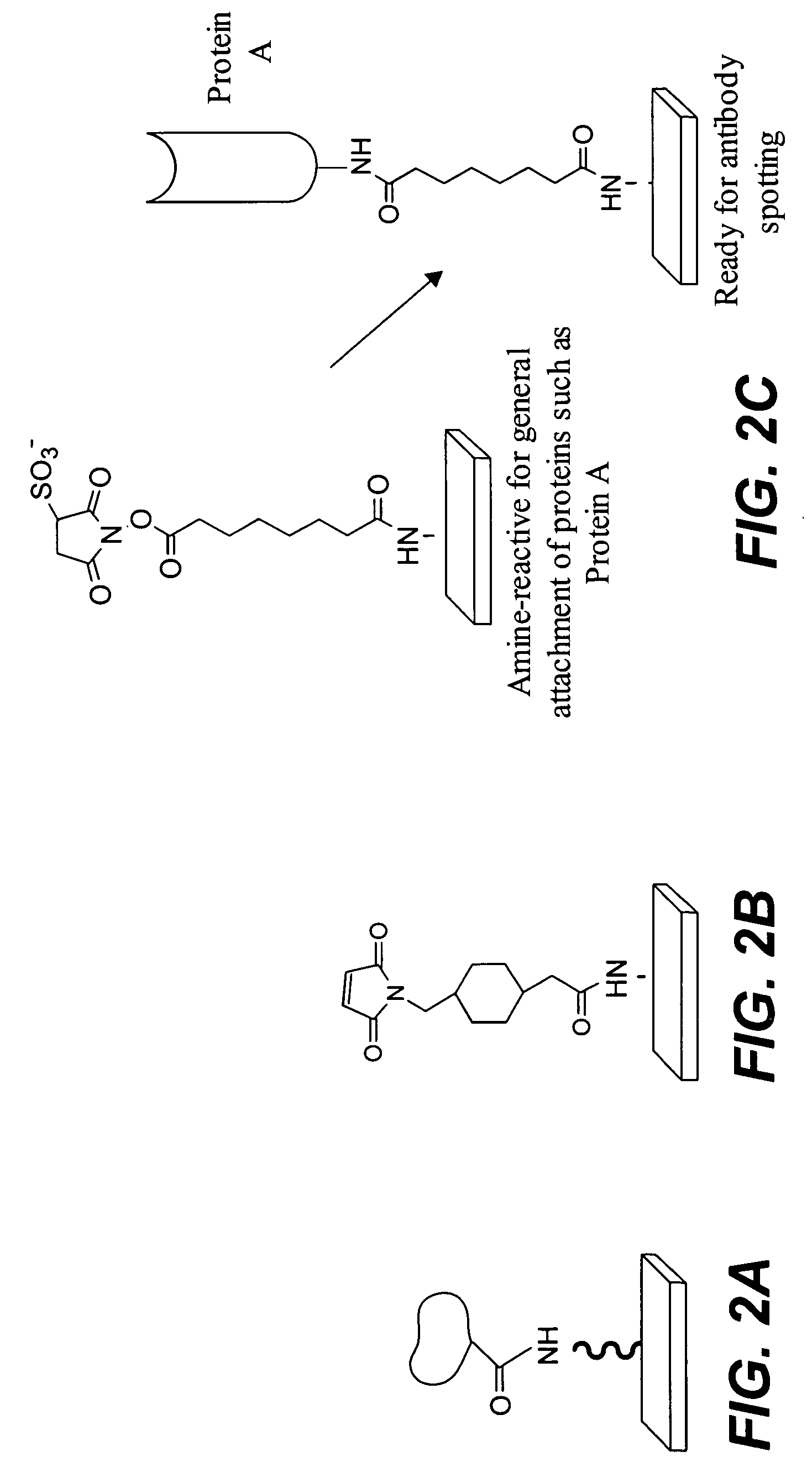
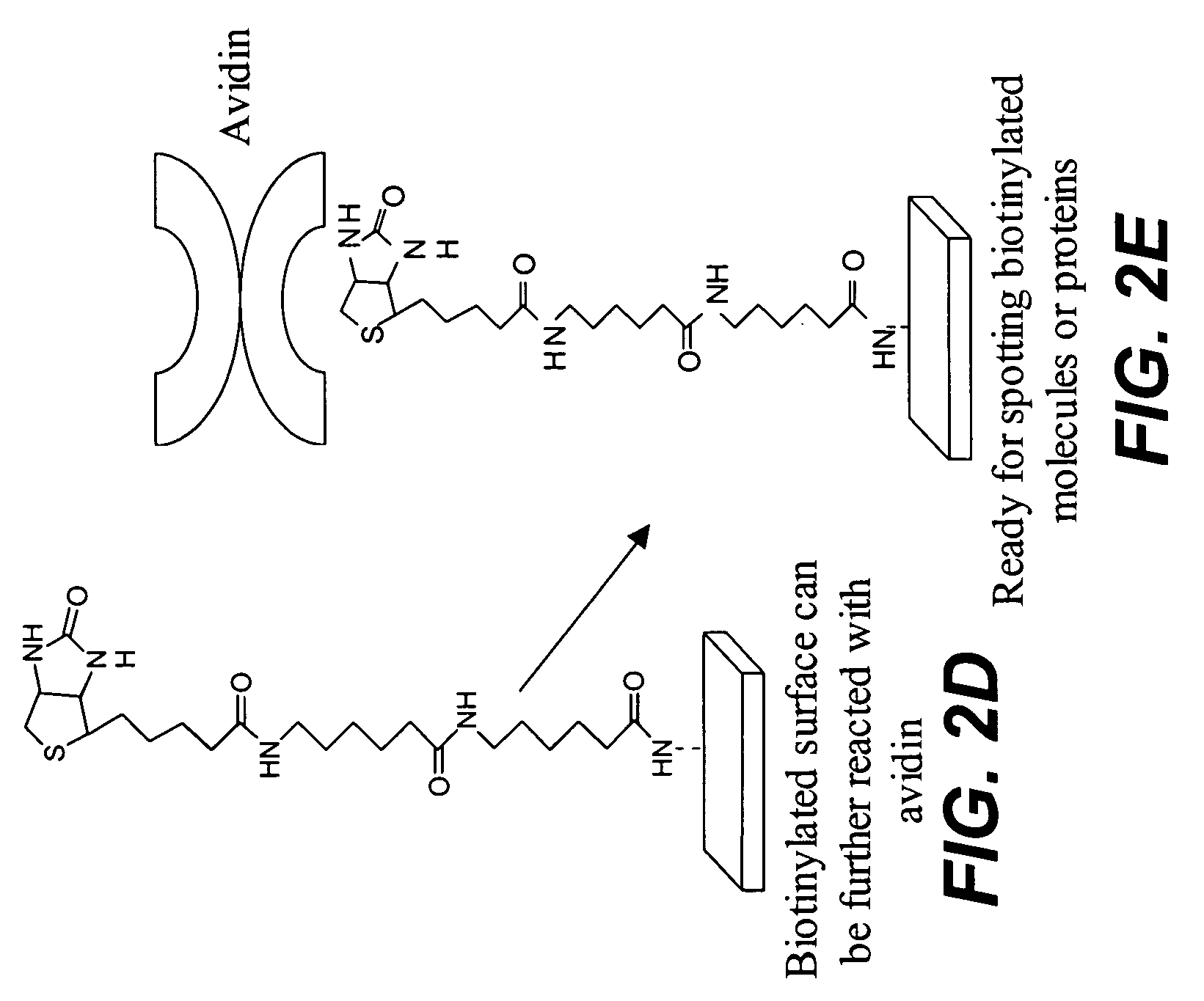
Protein microarrays on mirrored surfaces for performing proteomic analyses
a technology of protein microarrays and mirrored surfaces, applied in the field of cell product analysis and materials, can solve the problems of not providing sufficient blocking of non-specific protein binding, and achieve the effects of improving understanding of disease processes, facilitating drug discovery, and improving signal quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Method Used to Make Silanized Slides
[0135] Slide preparation: Glass microscope slides were pre-cleaned by sonicating in a soap bath for 10 minutes, followed by high pressure de-ionized water wash. The slides were further cleaned 5 min in Nochromix / H2SO4, rinsed with high pressure de-ionized water wash. Finally, they were immersed for 5 minutes in isopropyl alcohol and dried with a stream of N2. Pre-cleaned slides are also commercially available from, for example, Erie Scientific (Ultraclean) or Bioslide (Superclean).
[0136] E-beam: A CHA SEC-600-RAP e-beam was fitted with special wafer-sized inserts in the planetaries to accommodate the rectangular shape of the glass microscope slides. The e-beam crucibles were loaded with aluminum metal and fresh Si02. The chamber was then pumped down to at least 2×10−7 torr. After pumpdown, the planetaries containing the slides were rotated (to allow even coating) while the high voltage (about 10 V) was applied. The electron beam vaporized the ma...
example 2
Use of a Homobifunctional Adapter
[0138] Amine functionalized substrate surfaces were derivatized with an activated ester by reacting amine groups displayed on the substrate surface with the homobifunctional crosslinker bis-NHS ester. The NHS ester at one terminus of the adapter reacted with the amines on the substrate to produce an NHS ester functionalized slide as follows: 50 mg of adaptor was dissolved in 30 mL DMF, then 170 mL of 1×PBS or other suitable buffer was added. This was applied to the slides and incubated 1-2 hours with mixing. The slides were then rinsed in deionized water. The solution should be fresh to ensure that the NHS ester has not hydrolyzed.) Proteins containing exposed amine groups were spotted onto such a slide to produce covalently bound protein microarrays.
example 3
Use of a Heterobifunctional Maleimide Adapter
[0139] Amine functionalized substrate surfaces were derivatized with maleimide groups by reacting amine groups displayed on the substrate surface with heterobifunctional crosslinkers. SMCC (having an N-hydroxysuccinimide (NHS) ester at one terminus, and a maleimide group at the other) was applied using the same protocol described above in Example 2. The NHS ester reacted with the amines on the substrate to produce a maleimide functionalized slide. Proteins containing exposed thiol groups were spotted onto such a slide to produce covalently bound protein microarrays.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thick | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com