Liposome composition for improved intracellular delivery of a therapeutic agent
a technology of liposomes and compositions, applied in the direction of pharmaceutical delivery mechanisms, antibody ingredients, medical ingredients, etc., can solve the problems of the loss of the desired rapid destabilization of the liposome bilayer, and the accompanying rapid release of the entrapped agent into the cell. achieve the effect of increasing the accumulation of therapeutic agents and increasing the accumulation of agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
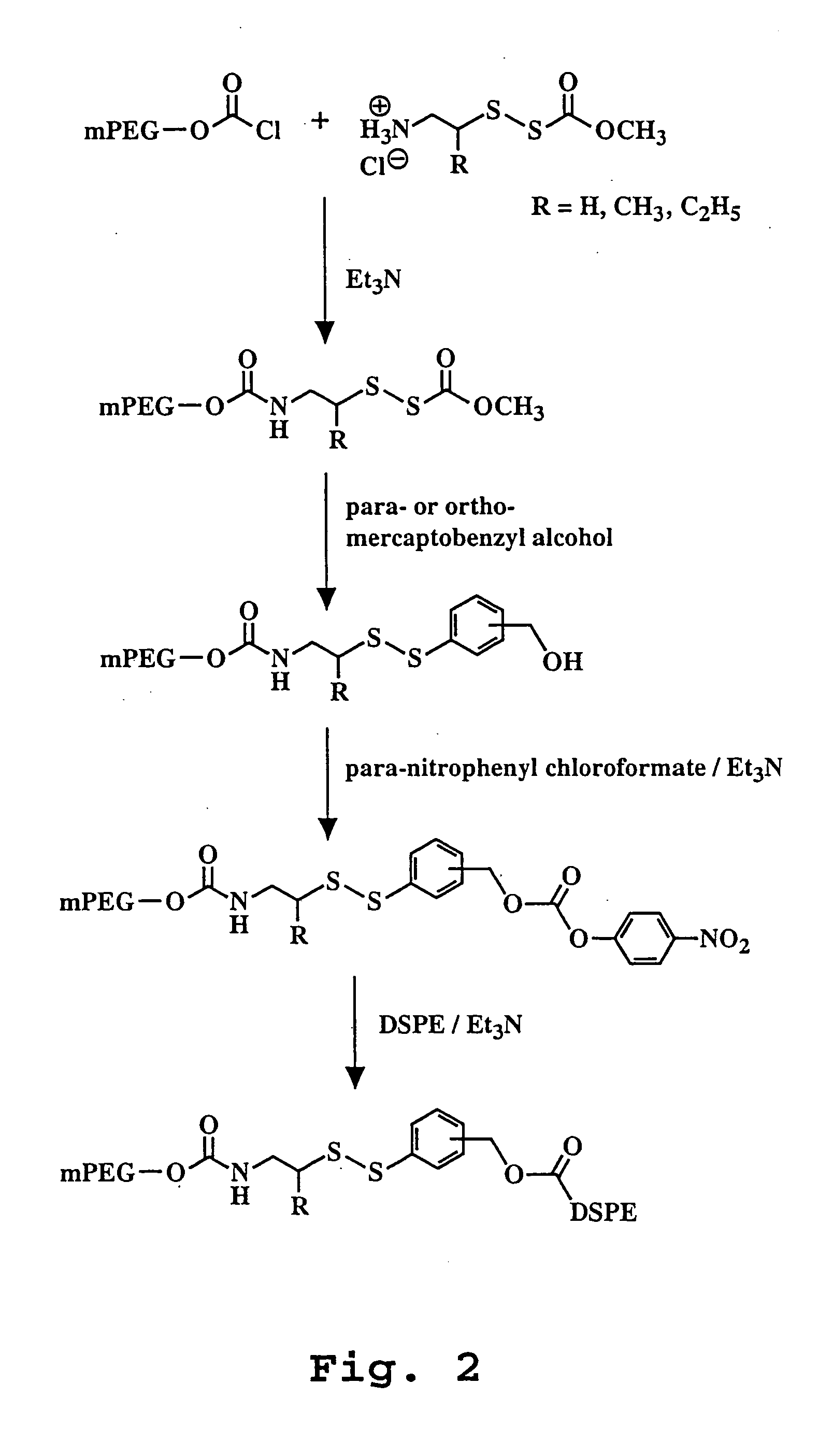
Image
Examples
example 1
Preparation of pH-Sensitive and Non-pH-Sensitive Liposomes
[0129] Sterically stabilized pH-sensitive liposomes were prepared from a mixture of dioleoylphosphatidylethanolamine (DOPE) or DOPE / CHEMS (cholesteryl hemisuccinate) and either mPEG-DSPE or mPEG-S-S-DSPE (prepared as described in Kirpotin et al.) and DSPE-PEG-maleimide (Mal-PEG-DSPE; prepared as described in U.S. Pat. No. 6,326,353) according to the lipid molar ratios of the desired formulations. The desired lipid mixture was dissolved in chloroform and dried as a thin film by rotation under reduced pressure using a rotary evaporator. The dried lipid film was hydrated by addition of an aqueous buffer to form liposomes. The liposomes were sized by sequential extrusion through a series of Nucleopore polycarbonate filters with pore size ranging from 0.2 to 0.08 μm, using a Lipex Extruder (Lipex Biomembranes, Vancouver, BC). The mean diameter of liposomes was determined by dynamic light scattering using a Brookhaven BI-90 Partic...
example 2
Antibody Coupling to Pre-Formed Liposomes
[0132] Coupling of anti-CD19 mAb to maleimide (Mal)-PEG-DSPE on the liposomes was carried out according to a previously described method (Lopes de Menezes et al., J.Liposome Res., 9:199 (1999)), using 125I-labeled anti-CD19 mAb as a tracer.
[0133] Antibodies were first activated with Traut's reagent (2-iminothiolane) at a molar ratio of 20:1 (Traut's: IgG), at a concentration of 10 mg IgG / ml buffer for 1 h at 25° C. in HEPES buffer, pH 8.0 (25 mM HEPES, 140 mM NaCl). Unreacted Traut's reagent was removed using a G-50 column. The coupling reaction was run at an IgG to phospholipid molar ratio of 1:2000, under argon atmosphere for 18 h at 25° C. Uncoupled Ab was removed from the liposomes by passing the coupling mixture through a Sepharose CL-4B column in HEPES buffer, pH 7.4. The coupling efficiency was on average 80%.
[0134] All mAb densities were routinely in the range of 30-60 μg anti-CD19 / μmol phospholipid for in vivo experiments and 65-8...
example 3
Leakage of Liposome-Entrapped Fluorescent Dye or Liposome-Entrapped Doxorubicin in Buffer
[0135] Leakage of entrapped HPTS-DPX from various formulations of DOPE or DOPE / CHEMS liposomes was evaluated by monitoring the release of entrapped solute using a fluorescence-dequenching assay. HPTS was passively loaded into the liposomes as the water-soluble (but fluorescence-quenched) complex, HPTS-DPX. When HPTS-DPX leaks from the liposomes; it dissociated into free HPTS and DPX, increasing the HPTS fluorescence when excited at 413 nM. The DOPE and DOPE / CHEMS formulations tested were:
Lipid ComponentsMolar RatioDOPE1DOPE / mPEG-DSPE1:0.05DOPE / mPEG-S-S-DSPE1:0.05DOPE / CHEMs6:4DOPE / CHEMS / mPEG-DSPE6:4:0.3DOPE / CHEMS / mPEG-S-S-DSPE6:4:03
[0136] Liposomes containing entrapped HPTS-DPX were passed over a Sephadex G-50 column immediately prior to use to remove any residual free dye or drug. Fifty (50) μl of liposomes containing entrapped dye (HPTS-DPX) or doxorubicin were incubated at 0.5 mM final PL c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com