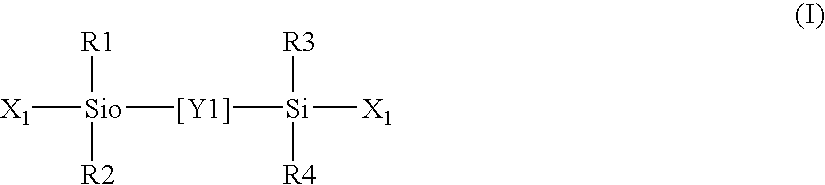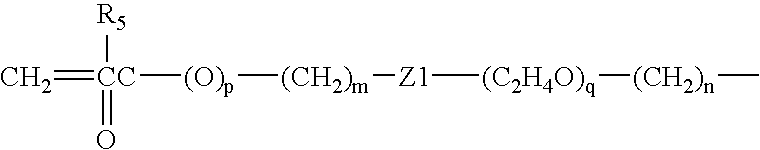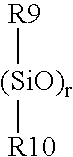Silicone hydrogel contact lens
a technology of silicone hydrogel and contact lens, which is applied in the field of contact lenses, can solve the problems of polarization techniques not providing accurate measurements of high-dk the surface wettability of existing silicone hydrogel contact lenses is not desirable, and the contact lens surface is more hydrophilic. , to achieve the effect of improving the properties of silicone hydrogel contact lenses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of Polysiloxanediol having Hydrosilane Groups (A1)
[0120] A mixture of 150 gms of octamethylcyclotetrasiloxane, 22.6 gms of 1,3,5-trimethyltrifluoropropyl-cyclotrisiloxane, 5.2 gms of 1,3,5,7-tetramethyl-cyclotetrasiloxane, 9.8 gms of 1,3-bis(3-(2-hydroxyethoxy)propyl)tetramethyldisiloxane, 200 gms of chloroform and 1.5 gms of trifluoromethane sulfonic acid was stirred for 24 hours at 25° C., then washed repeatedly with purified water until a pH of the mixture became neutral. After water was separated, chloroform was distilled off under the reduced pressure. The residual liquid was dissolved in acetone (36 gms), reprecipitated with methanol (180 gms), followed by removal of volatile components under vacuum from a separated liquid to give a transparent viscous liquid. The said liquid was the siloxanediol having hydrosilane groups expressed by the following formula (H3R) with a yield of 125 gms. Here, although the structural formula of the linking group Y is shown as a block...
synthesis example 1a
[0123] Synthesis Example 1 is repeated with appropriate adjustments to the amounts of the components and / or conditions utilized to provide a macromer structured similarly to M3-U except that Y has the following structure:
[0124] This material, identified as M3-UU, has a number average molecular weight of about 20,000.
synthesis example 2
[0125] A mixture of 50 gms of alpha-butyl-omega-[3-(2′ hydroxyethoxy)propyl)polydimethylsiloxane, 10 gms of methacryloyloxyethyl isocyanate, 150 gms of dry n-hexane and 0.2 gms of dibutyltin dilaurate was poured in a brown-colored flask and heated for 2 hours under reflux, then further stirred after an addition of 6 gms of methanol. Subsequently, n-hexane was distilled off under reduced pressure, and the resulting liquid was washed several times with methanol (30 gms) / water (15 gms) followed by removal of volatile components under vacuum to give a transparent viscous liquid with a yield of 54 gms. The liquid was the polysiloxane-methacrylate (FMM) expressed by the following formula.
This material, identified as FMM, has a number average molecular weight of about 1500.
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
| elongation | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com