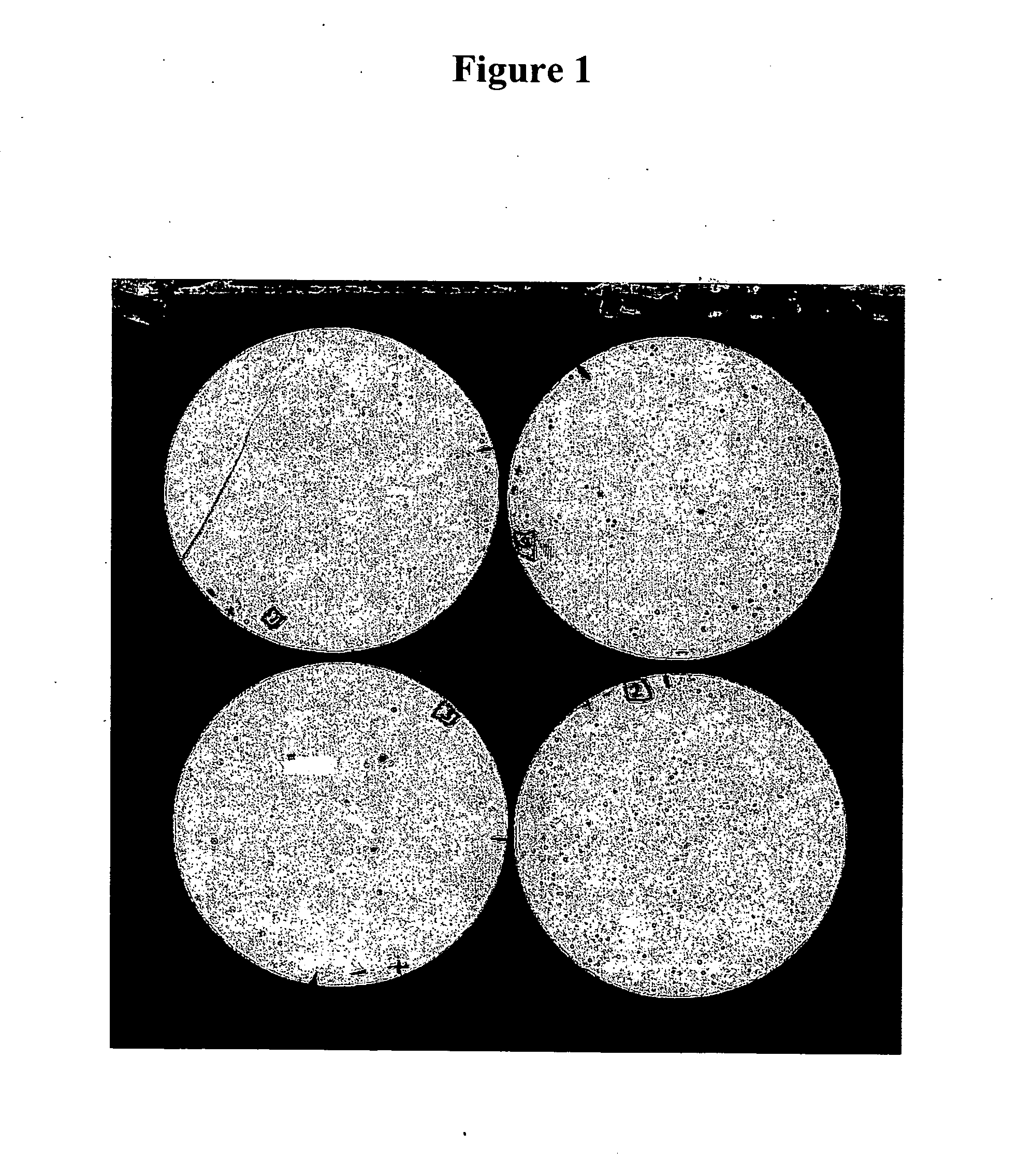
Compositions and methods for eliciting an immune response to gram-negative bacterial infections
a technology for eliciting an immune response and gram-negative bacteria, which is applied in the direction of bacterial antigen ingredients, peptides, medical preparations, etc., can solve the problems of limited production of glycosylated pilin having o-antigens, and achieves stronger immune response, longer lasting immune response, and improved purity and processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloned Serotype O11 O-Antigen Gene Cluster from P. aeruginosa PA103 Acts as O-antigen Source in P. aeruginosa 1244 LPS
[0139] In order to identify whether the O-antigen biosynthetic pathway serves as the source for the pilin glycan, a gene cluster encoding the O-antigen polysaccharides from serotype O11 of P. aeruginosa was moved into P. aeruginosa 1244 and tested for serotype-specific O-antigen expression on the LPS of this organism. The P. aeruginosa O11 O-antigen was chosen for the initial experiment because these genes have been isolated and characterized (Goldberg et al., 1992) and the O-antigen sugar residues are structurally very different from those found on the LPS of P. aeruginosa 1244. The P. aeruginosa O11 gene cluster is contained within a 15 Kb fragment on pLPS2 and was a gift from Dr. Joanna Goldberg (University of Virginia Health Sciences Center). This plasmid was conjugally transferred into P. aeruginosa 1244 by a triparental mating system described by Ruvkin and A...
example 2
Cloned Serotype O11 Gene Cluster from P. aeruginosa PA103 A cis as Pilin Glycan Source in P. aeruginosa 1244
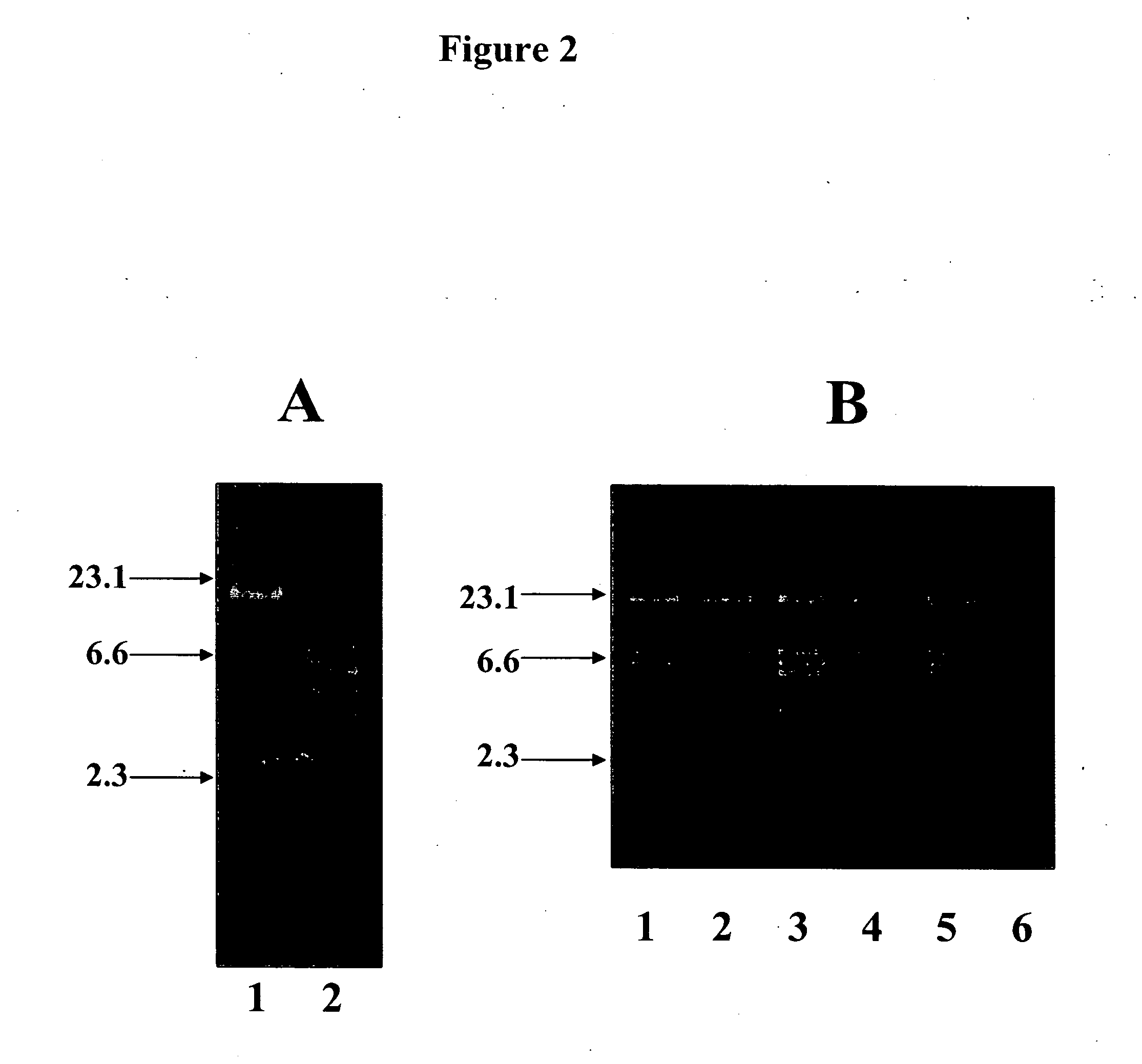
[0141] Once O11 O-antigen expression was established on the LPS of P. aeruginosa 1244 / pLPS2, the pili of this organism were tested for the presence of the O11 O-polysaccharides. Since the LPS O-antigen ladder will mask pilin analysis, proteins were separated electrophoretically by isoelectric focusing. LPS is a highly negatively charged structure and therefore migrate off of a focusing gel. Pilin proteins separated in this manner successfully removes the LPS. Pilin from P. aeruginosa 1244 / pLPS2 was extracted from overnight plate cultures and suspended in 1% n-octyl-p-D-glucopyranoside (BOG). BOG is a nonioniic detergent intended for solublizing membrane bound proteins in their native state, hence was used in this procedure. Pilin was separated by isoelectric focusing in a pH gradient of 3.0-9.0, transferred to PVDF membrane by diffusion blotting, and reacted with strain 1244...
example 3
The Cloned E. coli O157:H7 O-antigen Gene Cluster Acts as the O-Antigen Source for P. aeruginosa 1244 LPS
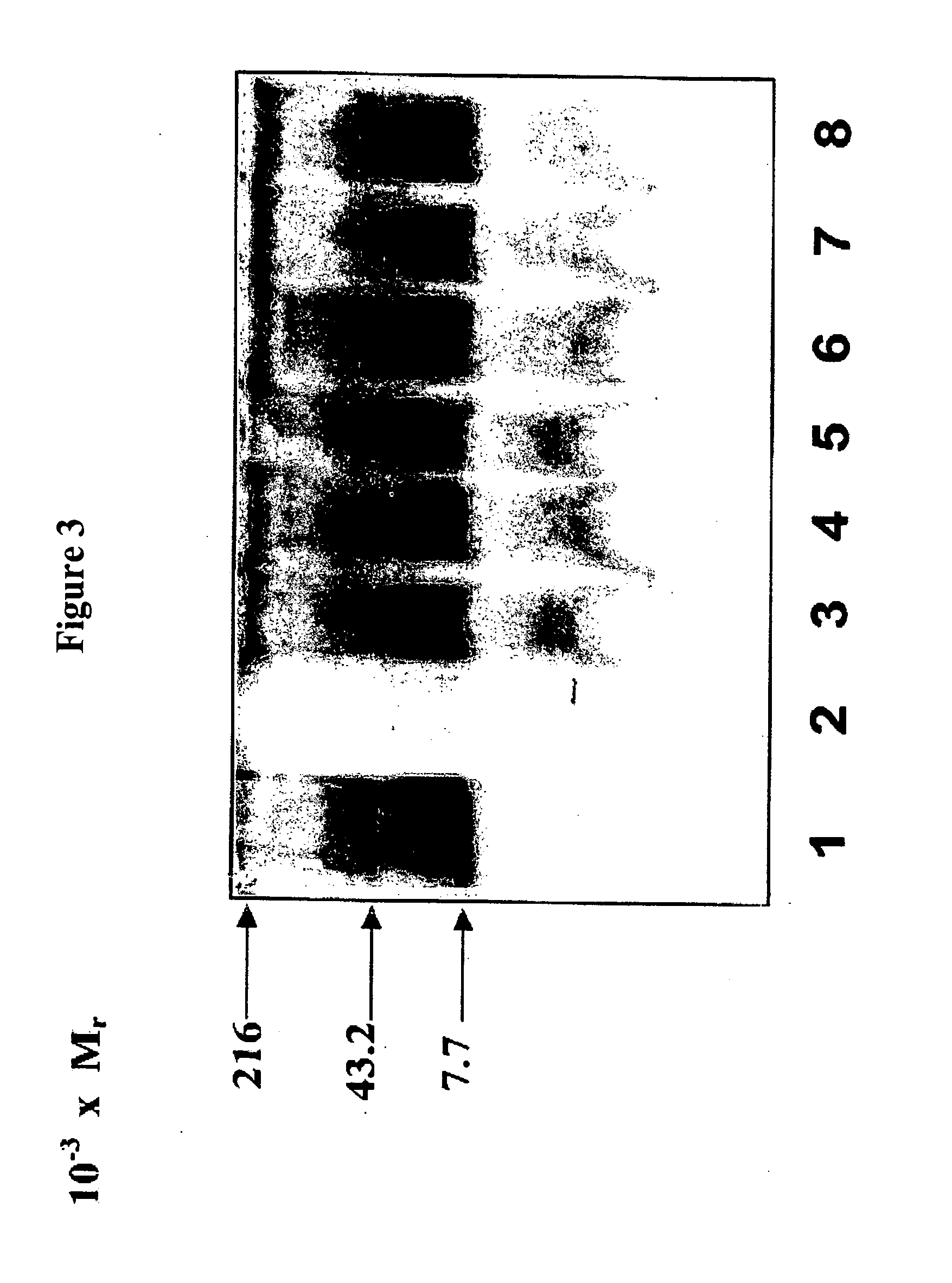
[0146] To identify whether the E. coli O157:H7 O-antigen could be expressed on P. aeruginosa 1244 LPS, pDIG4, one of the newly constructed cosmids, was transferred into P. aeruginosa 1244 and tested for O-antigen expression. As with P. aeruginosa 1244 / pLPS2, two colonies from this mating were chosen for further analysis. Initially, confirmation of pDIG4 incorporation into P. aeruginosa 1244 was accomplished by performing a plasmid extraction on each Tc resistant colony (results not shown). The LPS from each 1244 strain harboring the pDIG4 plasmid was then extracted from overnight broth cultures, and tested by Western blot using mAb 11.14 (FIG. 12, panel A) and the E. coli O157:H7 specific antiserum (FIG. 12, panel B). This figure shows that strain 1244 / pDIG4 produces the usual O-antigen ladder that is observed in the wild type 1244 strain when analyzed with mAb 11.14 (panel A, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com