Immunotoxin (mAB-RICIN) for the treatment of focal movement disorders
a technology of focal movement and immunonotoxin, which is applied in the field of immunonotoxin (mabricin) for the treatment of focal movement disorders, can solve the problems of increasing the risk of side effects, accompanied by repeated exposure to btx, and reducing the effect and duration of benefi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ITX Cytotoxic Activity
[0061] A. Protein Purification
[0062] Ricin was purified from seeds of Ricinus communis by elution from Sepharose columns with N-acetylgalactosamine, as described by Nicolson et al (Biochemistry (1974) 13:196-204). Onconase was purified from the eggs of Rana pipiens, as described previously (Ardelt et al., J. Biol. Chem., (1991) 266:245-251). CRM 107 (a mutated form of diphtheria toxin with an inactivated binding domain) was purified as described previously (Greenfield L, Johnson V G, Youle R J, Science, 1987;238:536-539). The plant lectin, RCA120, was purchased from Sigma (St. Louis,. MO). MoAb 35 (an anti-nicotinic acetylcholine receptor monoclonal antibody) (Tzartos et al., J. Biol. Chem., (1981) 256:8635-8645; Clementi and Sher, Eur. J. Cell Biol., (1985) 37:220-228; Tzartos et al., Proc. Natl. Acad. Sci. USA, 1982;79:188-192) was purified from ascites (mouse) by ammonium sulfate precipitation and DEAE sepharose.
[0063] B. Immunotoxin Synthesis
[0064] Conj...
example 2
Cytotoxic Activity Of Other Protein Toxins
[0077] In an effort to identify the most potent and specific reagent, three other toxins: RCA120, Tfn-CRM 107, and onconase were investigated. RCA120 (MW=120,000) is a tetrameric plant lectin similar to a dimer of ricin (Lin and Li, Eur. J. Biochem. (1980) 105:453-459). Myotubes and myoblasts were nearly equally sensitive to RCA120 (IC50=3.5×10−10 M). Tfn-CRM 107 (MW=150,000) is an immunotoxin (Johnson et al., J. Neurosurg. (1989) 70:240-248) selective for the transferrin receptor and high transferrin receptor numbers on myotube cell cultures and high rates of iron uptake (Sorokin et al., J. Cell Physiol. (1987) 131:342-353) have been observed. Tfn-CRM 107 was nearly as toxic to myotubes (IC50=3×10−7 M) as myoblasts (IC50=2×10−7 M). However, overall myotoxicity was lower than predicted. Onconase (MW=12,000), currently in phase III clinical trials for treatment of pancreatic cancer, was 2.5-fold more toxic to myotubes than myoblasts at the I...
example 3
Rat Muscle Biopsies
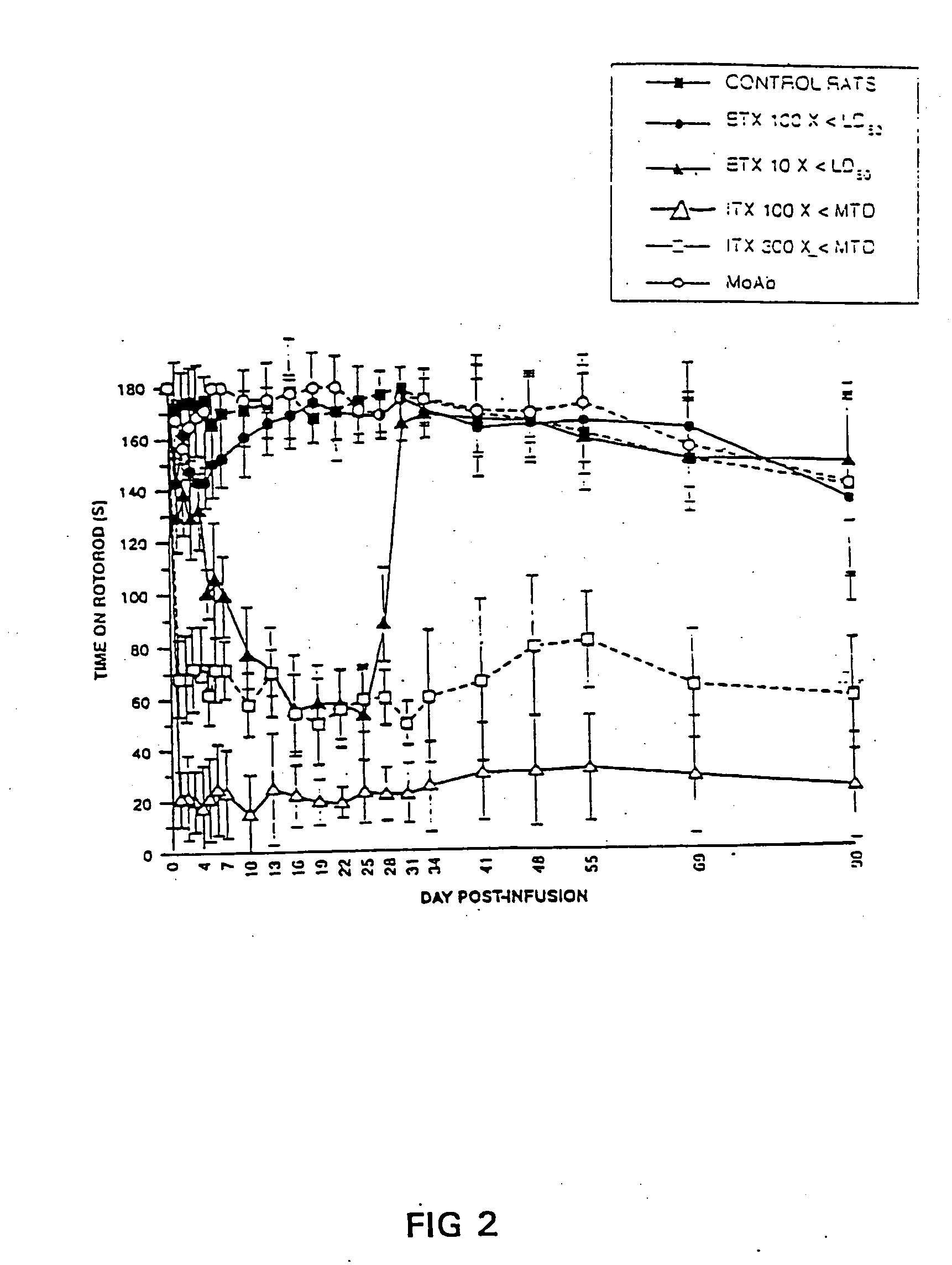
[0078] Female Balb / c mice (16-18 g) received 0.3 ml IP injections (30 g needle). For each agent, five serial 2-fold dilutions of toxin were tested. At each dilution three mice were injected and the experiment was repeated twice. The maximum tolerated dose was determined to be the maximum dose / kg where all animals survived. This dose was used as an estimate of the maximum tolerated dose (MTD) in rats. The maximum tolerated dose of ITX was 2 μg / kg in mice. Thus, for 250 g rats 1 / 100 and 1 / 300 of the maximum tolerated dose of ITX was estimated to be 5 ng and 1.7 ng, respectively. These doses of ITX were delivered intramuscularly to the rats in a volume of 30 μl. Hereafter in this example, comparable doses of the toxins refers to doses that are the same fraction of the maximum tolerated dose (or LD50 in the case of BTX). This comparison yields an estimate of the therapeutic window, which may be a useful gauge of the toxins clinical potential.
[0079] Frozen and lyophi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com