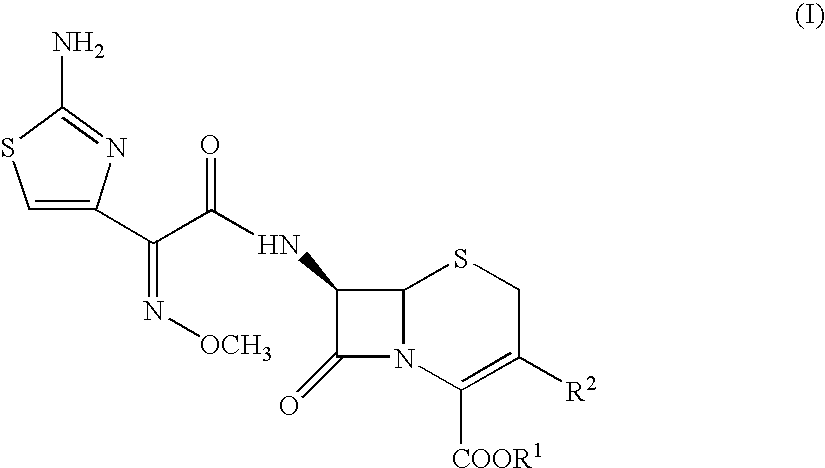
Process for preparing cephalosporins with salified intermediate
a technology of cephalosporins and intermediates, which is applied in the field of process for preparing cephalosporins with salified intermediates, can solve the problems of unsuitable industrial use methods, and achieve the effect of easy crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Sodium Ceftiofur
[0021] Two separate solutions are prepared.
Solution A
[0022] 40 g of FURACA (MW 340.38-0.118 moles) and 336 ml of tetrahydrofuran are fed into a dry 1 litre flask under a nitrogen flow in the absence of direct light. The mixture is agitated for 15 minutes until homogenization, while cooling in the meantime to +10° C.
[0023] While maintaining the temperature at +10° C.÷+12° C., 1.486 ml of trimethylchlorosilane (MW 108.64-d=0.859-0.1 eq) are quickly added. The mixture is agitated for 5 min at +10° C.÷+12° C., and 45.43 g of N,O-bis-trimethylsilyl-acetamide (MW 203.43-d=0.832-1.9 eq) are added over 5÷10 minutes.
[0024] The temperature is raised to +20° C. and the mixture agitated for 1 h35 min at 22° C.÷23° C. until a solution is obtained. It is cooled to −35° C.÷−40° C.
Solution B
[0025] 210 ml of ethyl acetate and 13.02 ml of N,N-dimethylformamide (MW 73.094-0.169 moles-d=0.95-12.37 g) are fed into a dry 1 litre flask under a nitrogen flow.
[0026] ...
example 2
Preparation of Ceftriaxone Disodium Salt
[0054] Two separate solutions are prepared.
Solution A
[0055] 15.57 g of 7-ACT (MW 371.39-0.042 mol) and 155 ml of methylene chloride are fed into a dry 1 litre flask under a nitrogen flow in the absence of direct light. The mixture is cooled to +10° C. and 34.11 g of N,O-bis-trimethylsilyl-acetamide are added, a slight amount of heat being produced. The mixture is agitated at +20÷22° C. and after 45 minutes a complete solution is obtained. The mixture is cooled to −40° C.
Solution B
[0056] 80 ml of ethyl acetate and 4.69 ml of N,N-dimethylformamide (MW 73.09, d=0.95) are fed into a dry 1 litre flask under a nitrogen flow at +25° C. 5.58 ml of phosphorus oxychloride (MW 153.33, d=1.675, 9.34 g) are added allowing the temperature to rise to 36° C. (if this temperature is not attained within 20÷25 minutes, heating is required). The mixture is cooled to 0° C. then 9.94 g of 4-chloro-3-oxo-2-methoxyimino-butyric acid, commonly known as COMBA (M...
example 3
Preparation of Cefotaxime Sodium Salt
[0064] Two separate solutions are firstly prepared.
Solution A
[0065] 64 g of 7-ACA (MW 272.28-0.235 mol) and 400 ml of tetrahydrofuran are fed into a dry 1 litre flask under a nitrogen flow and in the absence of direct light. The mixture is agitated for 15 minutes until homogenized while cooling to +15° C.
[0066] 191.34 g of N,O-bis-trimethylsilyl-acetamide (MW 203.43, d=0.832, 0.941 mol) are quickly added, maintaining the temperature at 20°÷25° C. The temperature is maintained at 20°÷25° C. while the mixture is agitated for 15 minutes at +20°÷+25° C. until dissolved, then cooled to −35° C.÷−40° C.
Solution B
[0067] 420 ml of ethyl acetate and 26.04 ml of N,N-dimethylformamide (MW 73.09, d=0.95, 0.338 mol, 24.74 g) are fed into a dry 1 litre flask under a nitrogen flow at +25° C. 30.98 ml of phosphorus oxychloride (MW 153.33, d=1.675, 51.9 g) are added allowing the temperature to rise to 36° C. (if this temperature is not attained in 20÷25 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com