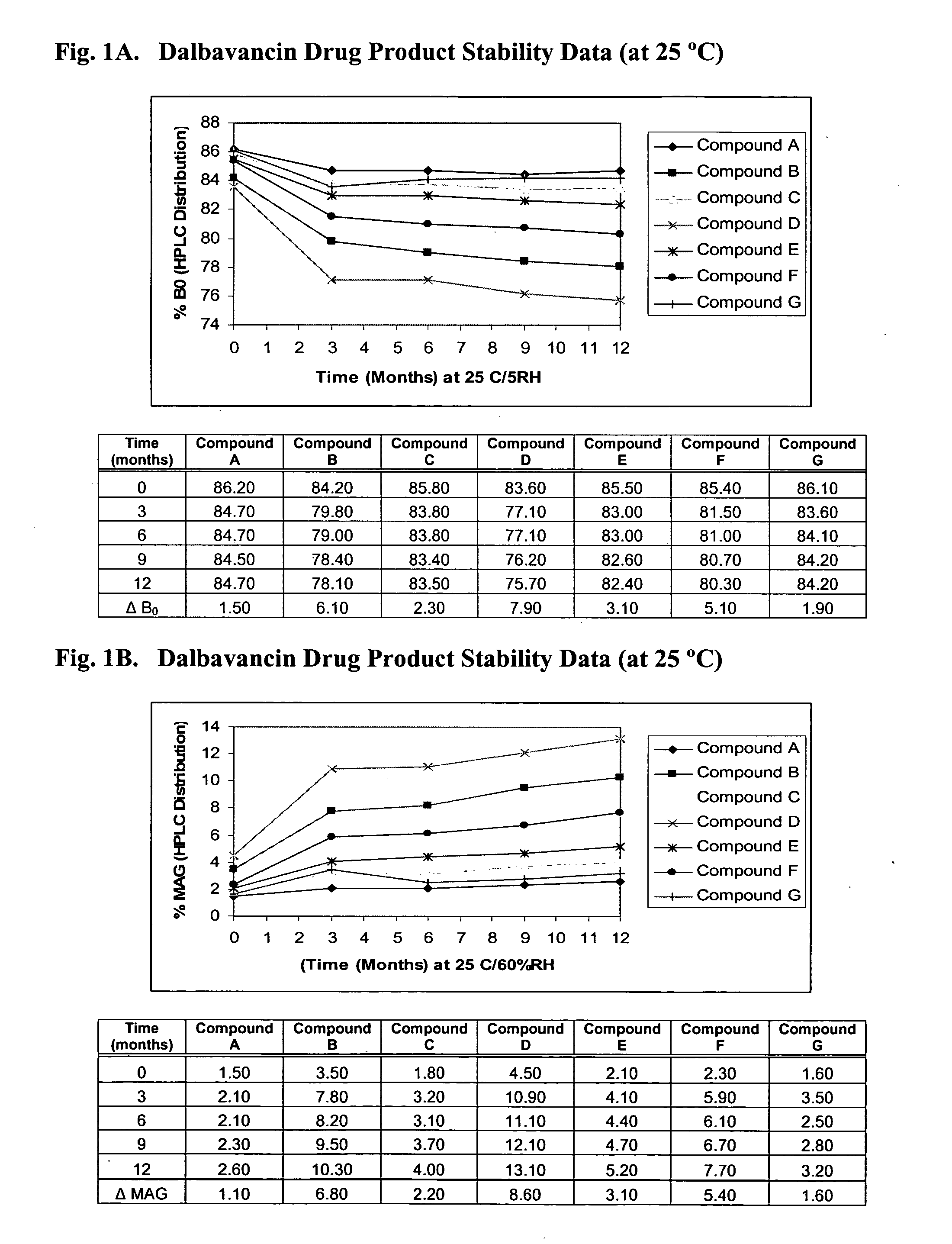
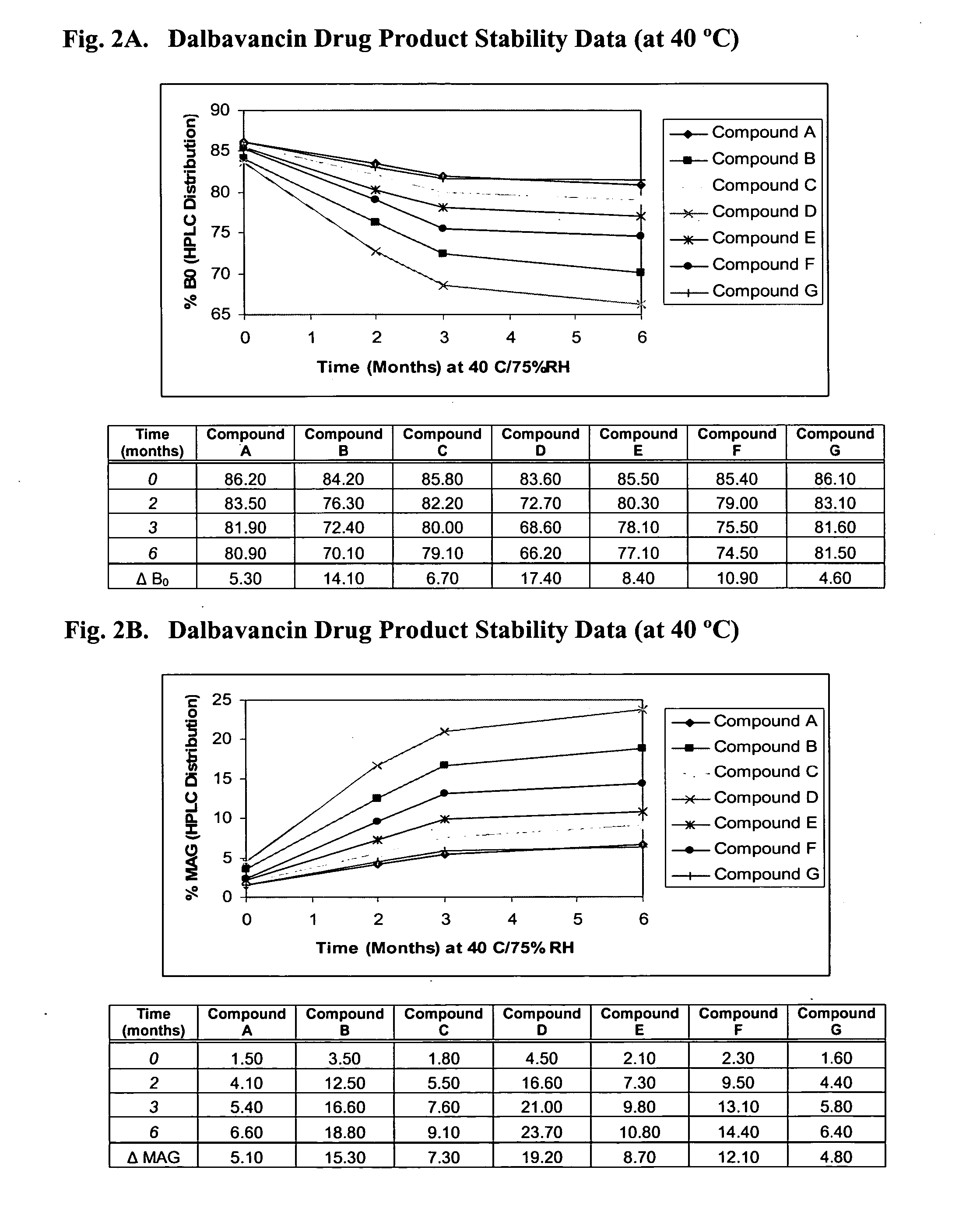
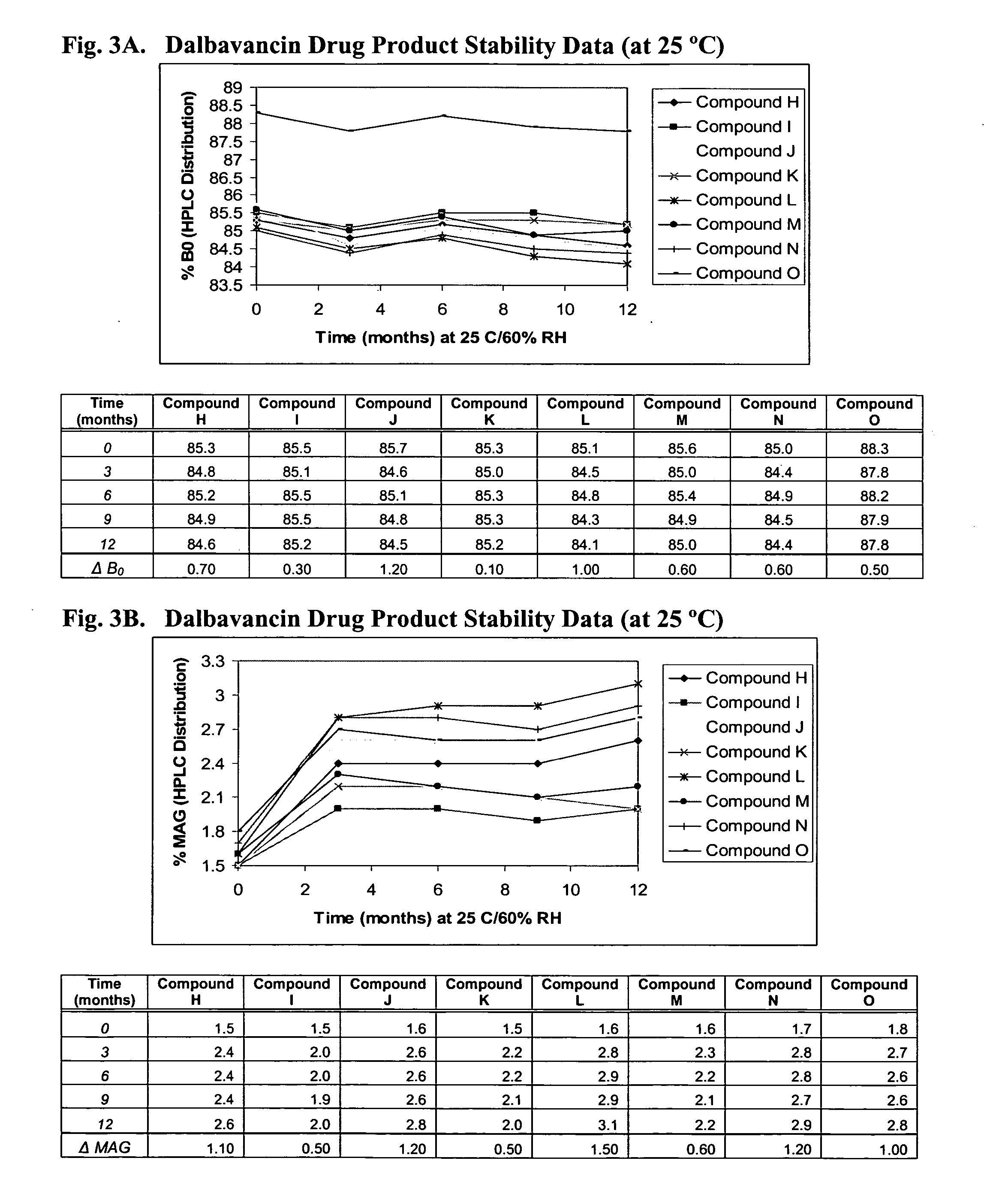
Methods for treatment of bacterial infections in impaired renal patients
a technology of bacterial infections and renal impairment, applied in the field of dalbavancin compositions, can solve the problems of life-threatening, limited treatment options for severe infections caused by some of these pathogens, and less frequent dosing, and achieve the effect of reducing adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Efficacy and Safety of Once Weekly, Dalbavancin in Deep Skin and Soft Tissue Infections
This randomized, controlled study evaluated the safety and efficacy of two dose regimens of dalbavancin. Adult patients with skin and soft tissue infections (SSTI) involving deep, skin structures or requiring surgical intervention were randomized to three groups: Study arm 1 received 1100 mg of dalbavancin via intravenous injection (IV) on day 1; Study arm 2 received 1 g of dalbavancin IV on day 1 and 500 mg of dalbavancin IV on day 8; Study arm 3 received “standard of care.” Clinical and microbiological response and adverse events were assessed.
Populations for Analysis
There were 62 patients randomized into the study; all received at least one dose of study medication. Four study populations were evaluated for safety and efficacy and were defined as follows: The intent-to-treat (ITT) population included all patients who received at least one dose of study drug (all randomized study subjects)...
example 2
Efficacy and Safety of once Weekly Dalbavancin in the Treatment of Catheter-Related Blood Stream Infection (CR-BSI)
This study evaluated the safety and efficacy (clinical and microbiological) of a weekly dosing regimen of dalbavancin in the treatment of adults with catheter-related blood stream infection (CR-BSI) due to gram-positive bacterial pathogens, relative to the standard care of treatment, vancomycin.
Methodology
Patients with CR-BSI due to suspected or known Gram-positive pathogen(s) who met the inclusion / exclusion criteria were randomized to one of two treatment arms in this open-label, comparative, multi-center study. Dalbavancin was administered once weekly in weekly dalbavancin and comparator (vancomycin) was administered daily in vancomycin. Catheter removal was required for patients with Staphylococcus aureus infection; catheter management was left to the Investigator's discretion for patients with coagulase-negative staphylococci (CoNS) infection. Efficacy was cli...
example 3
Pharmacokinetics and Renal Excretion of Dalbavancin in Healthy Subjects
The primary objectives of this study were to characterize the pharmacokinetics of dalbavancin and to calculate the extent of renal excretion in healthy subjects receiving a therapeutic dose of the drug. This was an open label, non-comparative, study.
Study Drug Treatment
Healthy male or female subjects between 18. and 65 years of age were administered a single 1000 mg IV dose of dalbavancin infused over 30 minutes.
Six subjects, one female and five male, were enrolled, received study medication, and completed all aspects of the study. Three subjects were Caucasian and three subjects were African-American. Mean age was 29.8 years (range 22 to 63). Mean height was 68.6 inches (range 63 to 75) and mean weight was 179.6 lbs (140 to 244).
Blood and urine (24-hr collections) were collected on study days 1, 2, 3, 4, 5, 6, 7, 14, 21, 28, and 42. Blood samples were drawn into heparinized tubes and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com