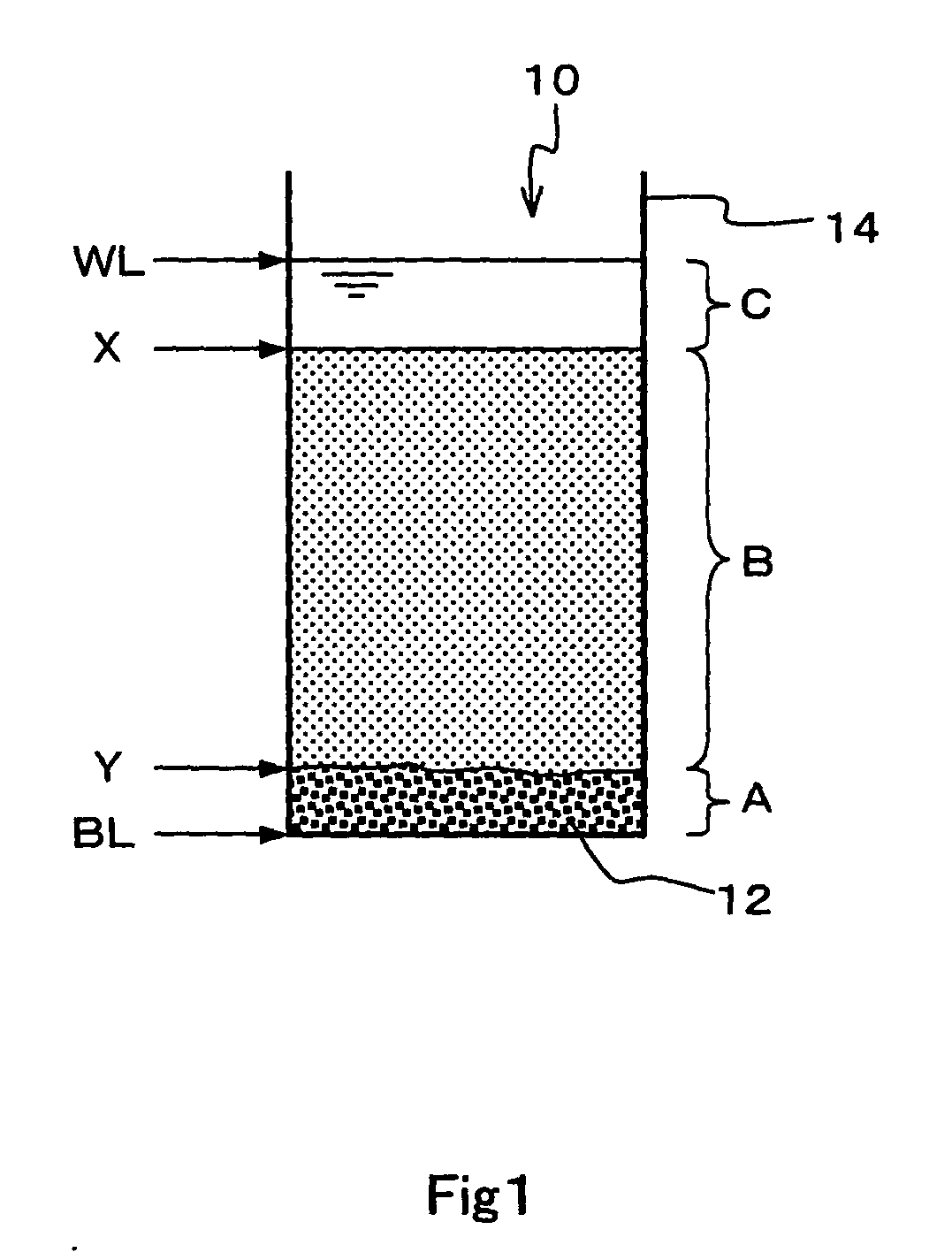
Carbon nanotube dispersion liquid and method for producing the same and polymer composite and method for producing the same
a technology of carbon nanotubes and dispersion liquids, which is applied in the field of carbon nanotube dispersion liquids and methods for producing the same, can solve the problems of insufficient development of the cylindrical network structure of carbon fibers to be used as structural materials with a diameter of several micrometers or more, the number of amorphous carbons being attached, and the failure of the manufacture of carbon nanotubes having the same structur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0108] Materials:
[0109] (a) MWNT (95% in purity, manufactured by Science Laboratory Co., Ltd.) . . . 0.02 g
[0110] (b) Concentrated nitric acid (60% by mass) . . . 14 g
[0111] (c) Pyridine
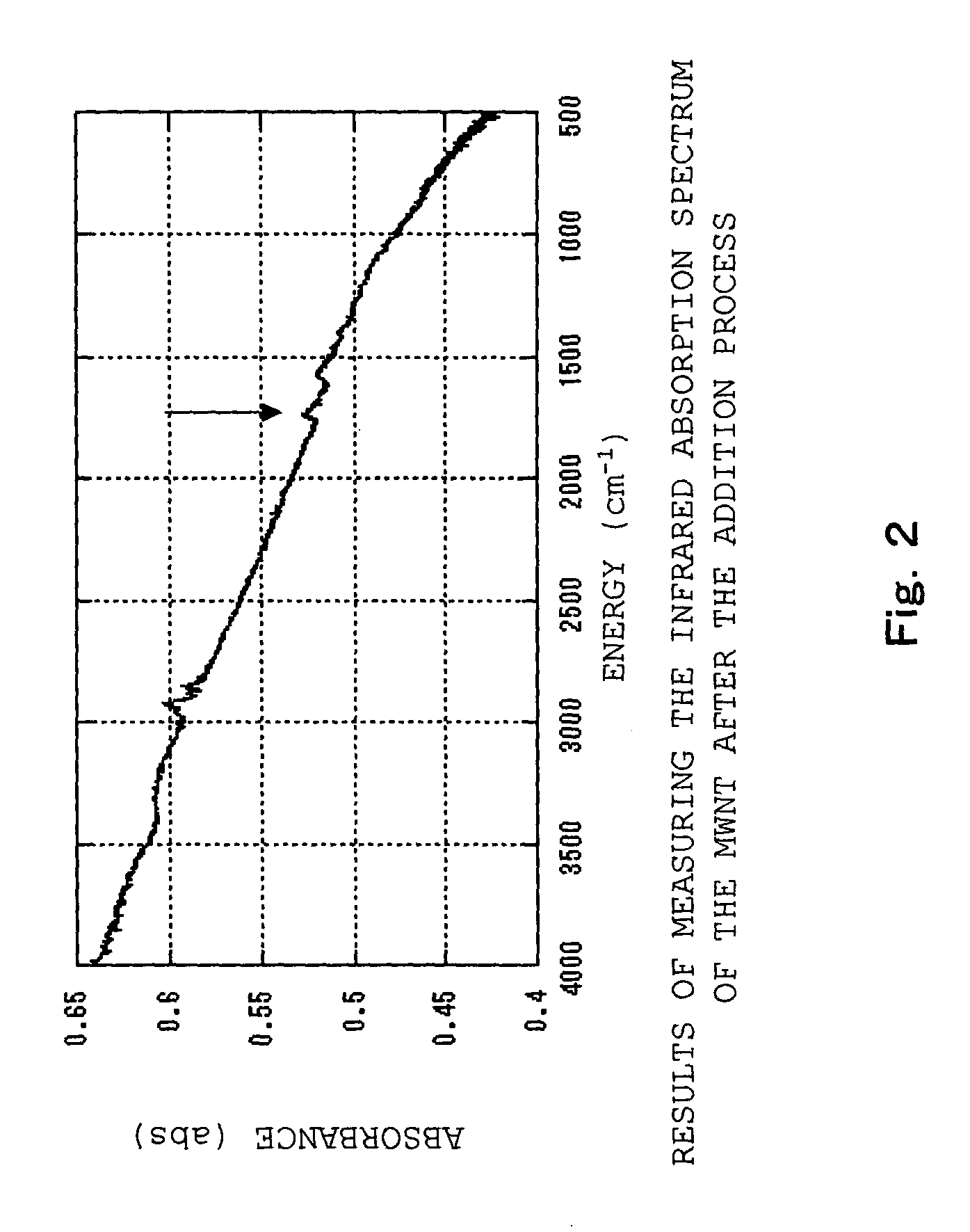
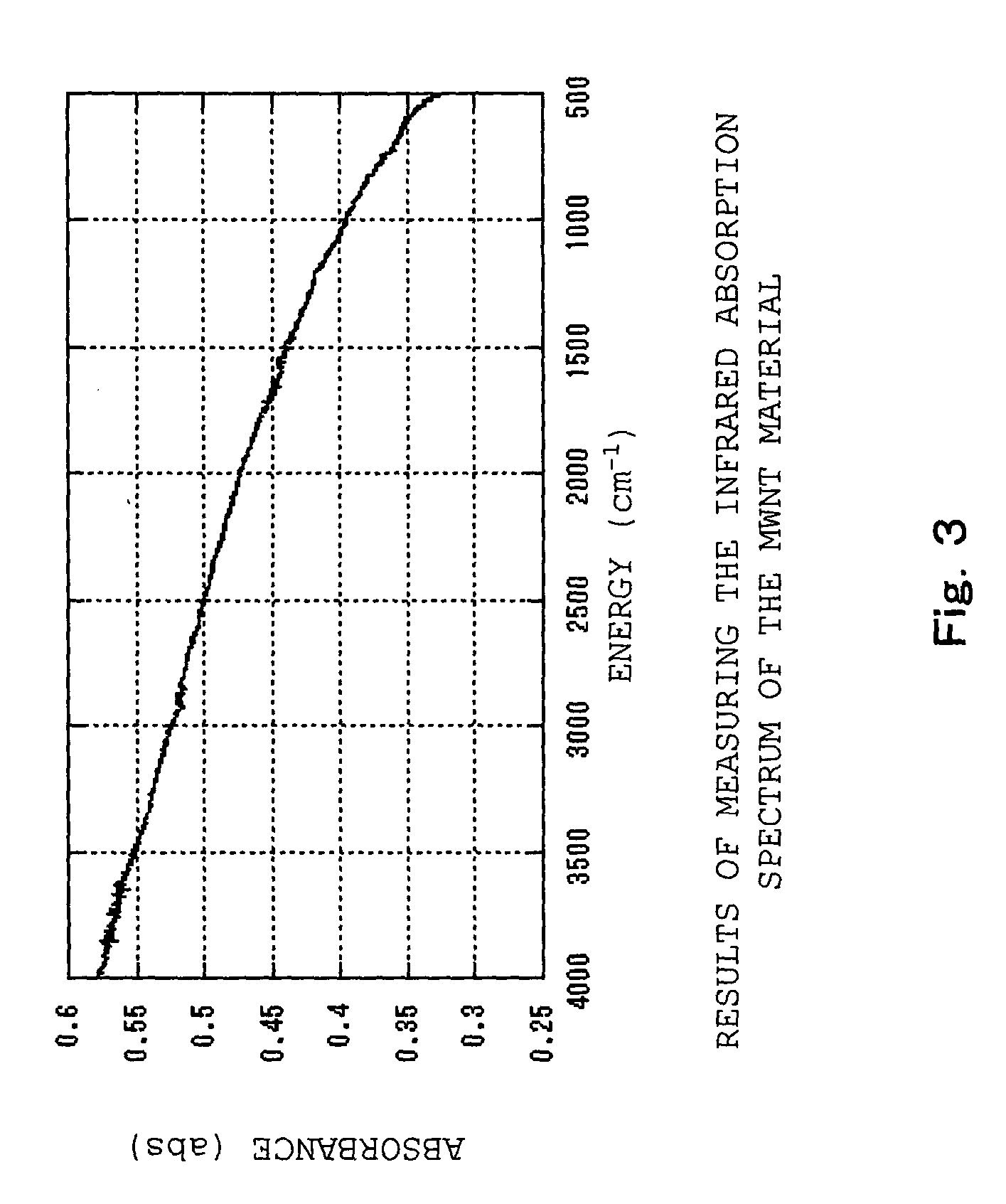
[0112] First of all, a carbon nanotube was ground with a mortar for 5 minutes in advance to make the surface of the carbon nanotube to be easily reacted, and then a mechano-chemical force was applied thereon. The carbon nanotube was added to the concentrated nitric acid and then the mixture was refluxed in an oil bath (120.degree. C.) for 4 hours. After that, centrifugation and decantation were repeated until the pH of the supernatant shifted to neutral (pH=6 or more), Subsequently, the resulting dispersion liquid was dried up and the modified carbon nanotube (aggregation) was obtained (the addition process so far). Here, the carboxyl group of the modified carbon nanotube released as the pH shifted to neutral that increases dispersibility of the carbon nanotube. Therefore, in the present example, cen...
example 2
[0117] Materials:
[0118] (a) MWNT (95% in purity, manufactured by Science Laboratory Co., Ltd.) . . . 0.02 g
[0119] (b) Concentrated nitric acid (60% by mass) . . . 14 g
[0120] (c) Pyridine
[0121] First of all, in the same manner as in Example 1, a mechano-chemical force was applied to a carbon nanotube. The carbon nanotube was added to the concentrated nitric acid and then the mixture was refluxed in an oil bath (120.degree. C.) for 4 hours. After that, in the same manner as in Example 1, when the pH of the supernatant shifted to neutral, the resulting dispersion liquid was dried and then the aggregation of a carbon nanotube carboxylic acid was obtained (addition process so far).
[0122] The obtained condensation of the carbon nanotube carboxylic acid was added to pyridine so as to be 0.1% by weight, and then the mixture was dispersed in the same manner as in Example 1. Consequently, a carbon nanotube dispersion liquid of Example 2 was prepared (Dispersion process, so far).
[0123] FIG. 5 ...
example 3
[0139] Materials:
[0140] (a) MWNT (95% in purity, manufactured by Science Laboratory Co., Ltd.) . . . 0.02 g
[0141] (b) Concentrated nitric acid (60% by mass) . . . 14 g
[0142] (c) U-Varnish-A (A 20% by mass solution of polyimide precursor in N-methyl pyrrolidone, manufactured by Ube Industries, Co., Ltd.) . . . 0.56 g
[0143] (d) Pyridine
[0144] (Preparation of Mixture Solution)
[0145] The carbon nanotube dispersion liquid (1 g) obtained in Example 1 was mixed with 0.56 g of U-Varnish-A and the resultant solution was stirred well with a magnetic stirrer, followed by defoaming the resulting mixture in vacuum for 60 minutes to prepare a mixture solution.
[0146] (Volatilization)
[0147] The obtained mixture solution was used and about 0.5 ml of the mixture solution was dropped on one side of a glass substrate using a Pasteur pipette to form a cast coat. Then, the glass substrate was heated at 210.degree. C. for 60 minutes to volatilize the solvent, forming a film on the glass substrate. Further...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com