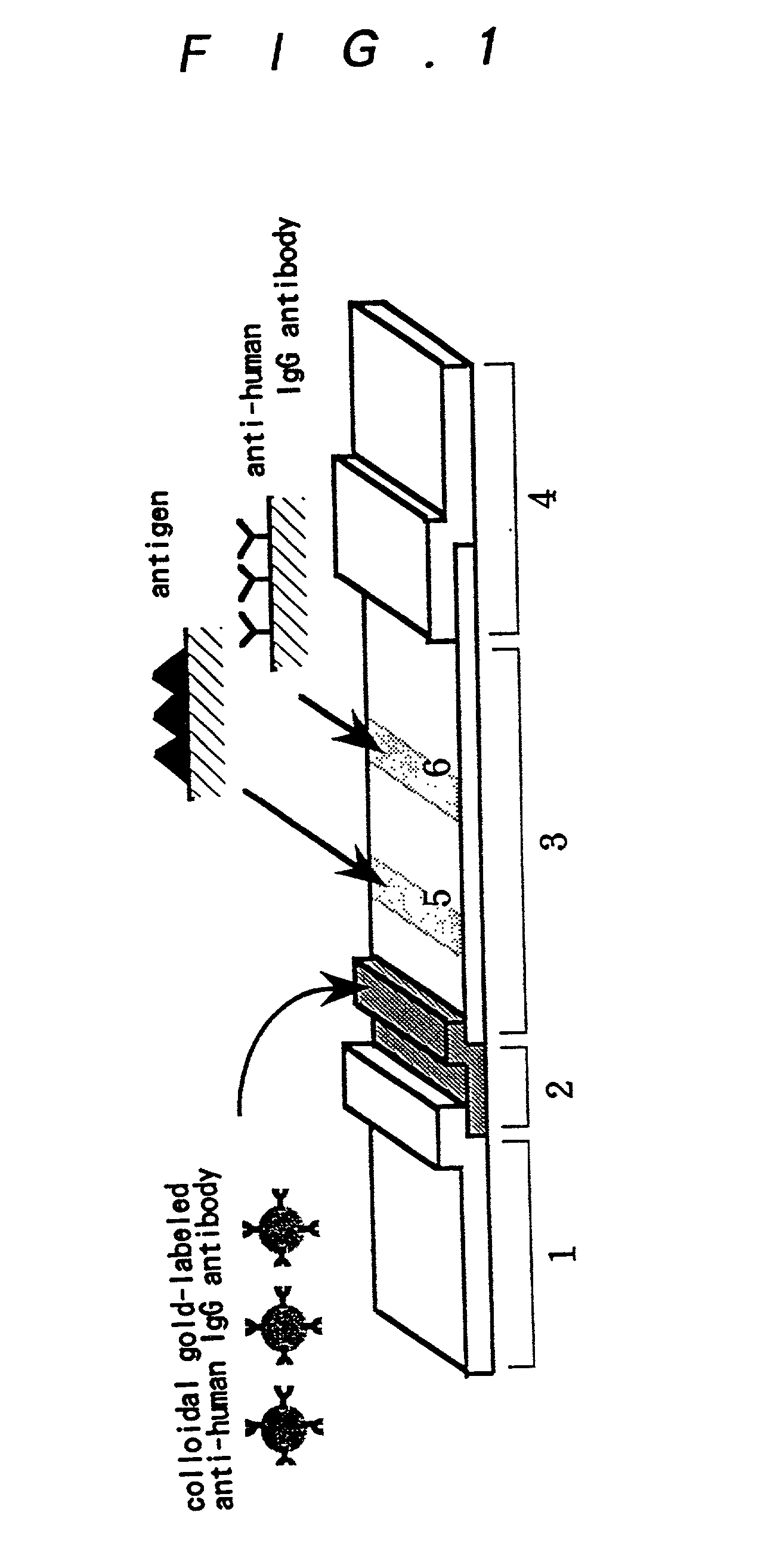
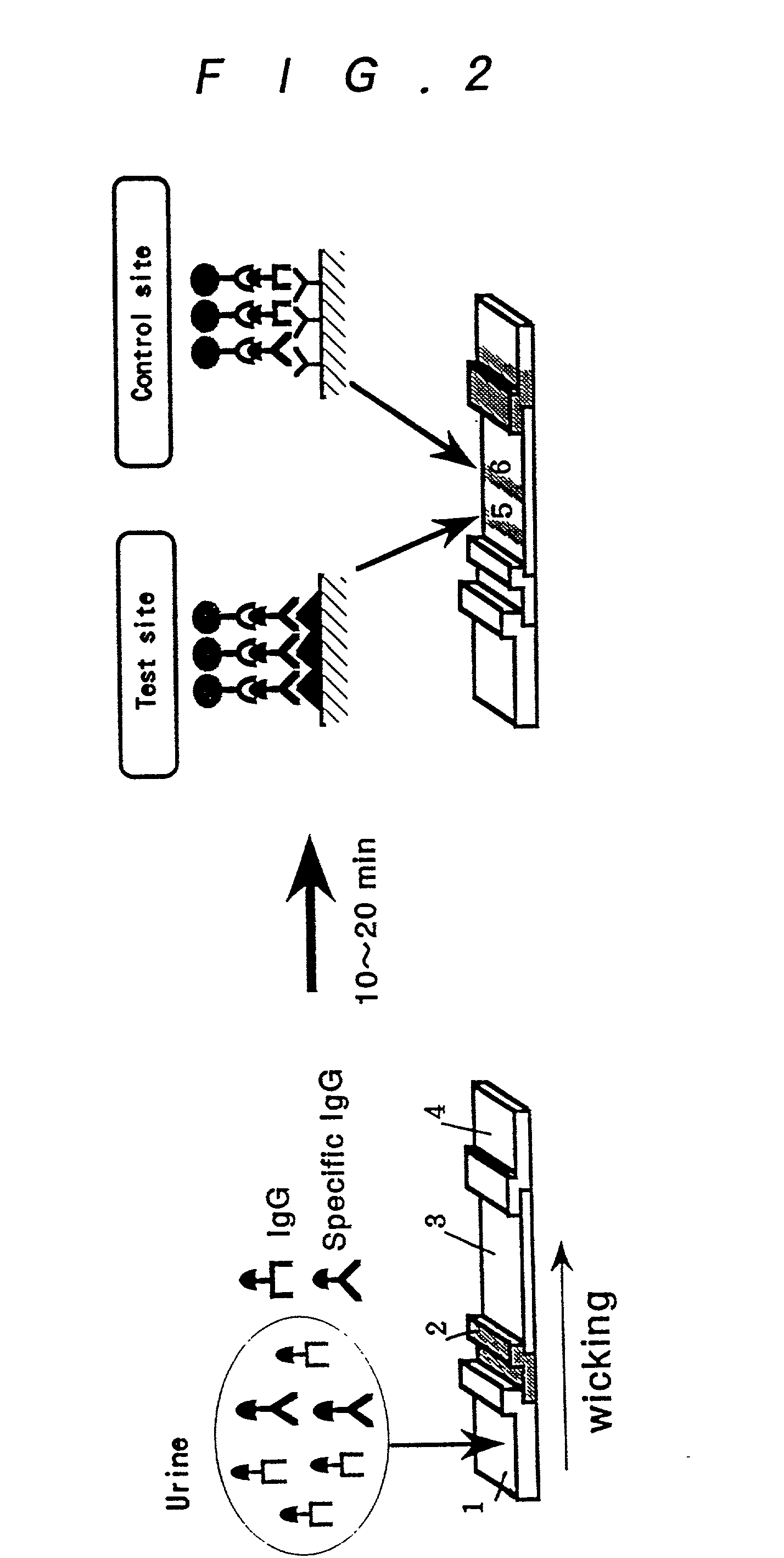
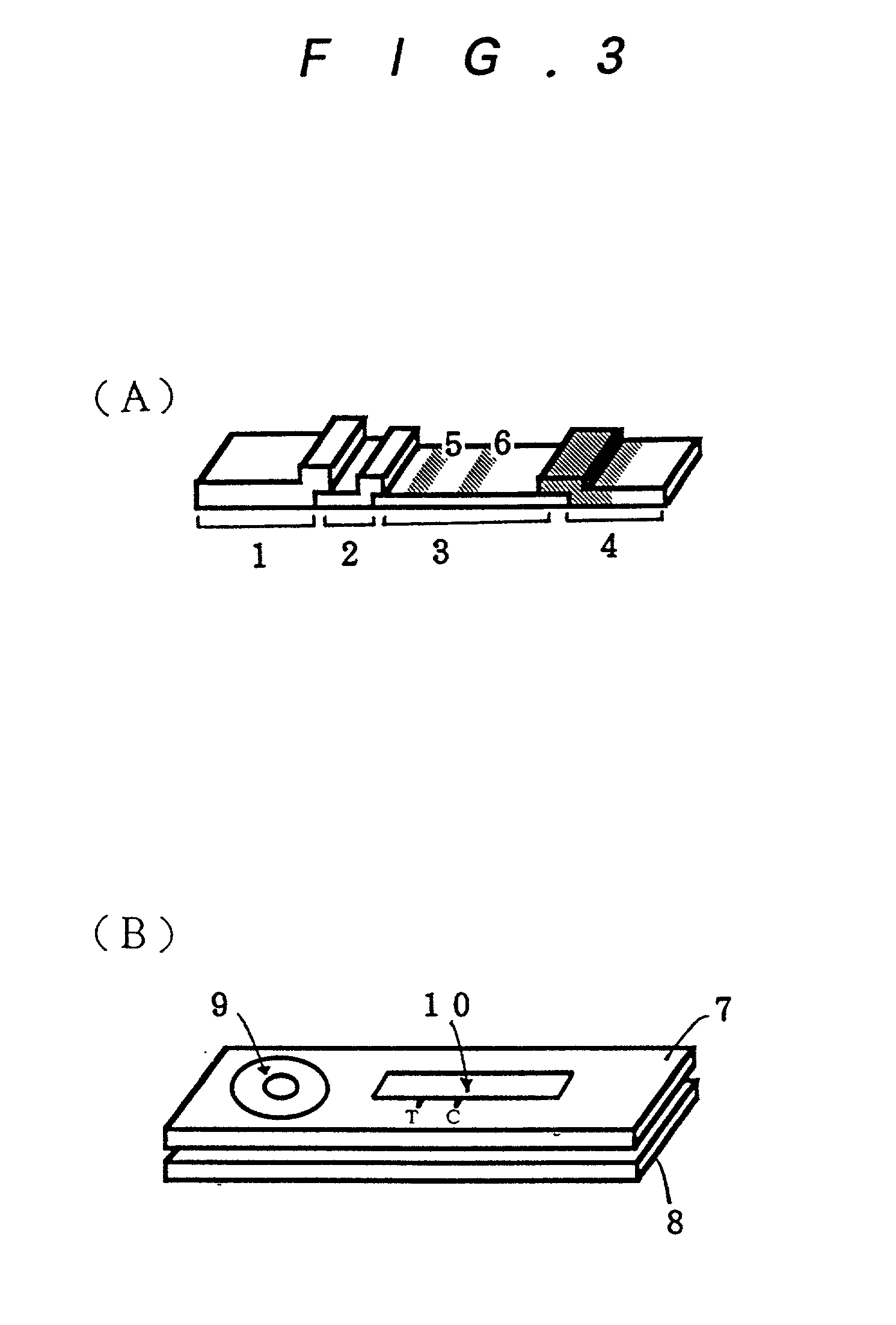
Method for assay of antibodies and antibody assay device
a technology of antibodies and assay devices, applied in the field of antibodies and antibody assay devices, can solve problems such as false positive tests
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Assay of Anti-hepatitis B Virus (HBc) Antibody
[0223] (1) Assay of Anti-hepatitis B Virus (HBc) Antibody
[0224] An antigen plate was prepared using HBc antigen (Chemicon International) in accordance with the procedure of Example 1 (2) and the assay of anti-hepatitis B (core) (HBc) antibody in urine samples was carried out in accordance with Example 1 (4).
[0225] Thus, 25 .mu.l of first buffer containing the E. coli extract in a varying concentration and 100 .mu.l of sample urine were added to each well of the HBc antigen plate and after 10 seconds' stirring, the plate was allowed to sit at 37.degree. C. for 1 hour. After the plate was washed with 6 portions of PBST, 100 .mu.l of a 11,000-fold dilution of enzyme (HRP)-labeled anti -human IgG antibody in second buffer was added and the plate was allowed to sit at 37.degree. C. for 1 hour and then washed (6 times with PBST).
[0226] Then, 100 .mu.l of a color developer solution was added and the reaction was carried out at room temperature ...
example 3
[0231] Using urine samples which gave false positive tests in the determination of anti-HIV antibody in urine by a known assay method [Calypte.TM. HIV-1 Urine EIA: Arch. Pathol. Lab. Med., 119, 139-141 (1995); Clinical Infectious Diseases, 19, 1100-1104 (1994)], an exploratory experiment was carried out to identify the component supposedly responsible for a nonspecific reaction in the same assay system.
[0232] (1) Each of the above urine samples was adjusted to pH 7.4 with 1 M phosphate buffer (pH 7.7) and filtered through 5.0, 0.8 and 0.2 .mu.m-cut filters. A 20 ml portion of the filtrate was concentrated by ultrafiltration (a 10 kDa-cut membrane) to 2 ml. The concentrated urine was subjected to gel permeation chromatography (Sephacryl S-300, Pharmacia) and each fraction was tested for its reactivity to HIV antigen.
[0233] The reactivity to HIV antigen was confirmed by causing each fraction to react with an HIV antigen-immobilized plate prepared by immobilizing HIV antigen (gp160) an...
example 4
Assay of Anti-HIV Antibody in Urine
[0237] To each well of an HIV antigen plate (Calypte.TM. HIV-I Urine EIA, Calypte Biomedical Corp.), 25 .mu.l of first buffer (the sample buffer of Calypte.TM. HIV-I Urine EIA) and 200 .rho.l of sample urine were added, and after 10 seconds' stirring, the plate was allowed to sit at 37.degree. C. for 1 hour. After this plate was washed 6 times (wash buffer: D-PBS, 0.05% Tween 20), 100.mu. 1 of a 11,000-fold dilution of HRP-labeled goat anti-human IgG (Fc-specific) antibody (Peroxidase-conjugated Affini Pure Goat anti-Human IgG, Fc Fragment Specific, Jackson ImmunoResearch Labs.) in second buffer (50 MM Tris-HCl buffer, 0.14M NaCl, 0.5% BSA, 5% Goat Serum, 0.05% Tween20, 0.1% XL-II (pH 7.3)) was added and the plate was allowed to sit at 37.degree. C. for 1 hour and washed (6 times) in the same manner as above.
[0238] Then, 100 .mu.l of a color developer (50% TMB solution, 50 mM Citrate-Na.sub.2HPO.sub.4, 0.0075% H.sub.2O.sub.2) was added and reacted ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com