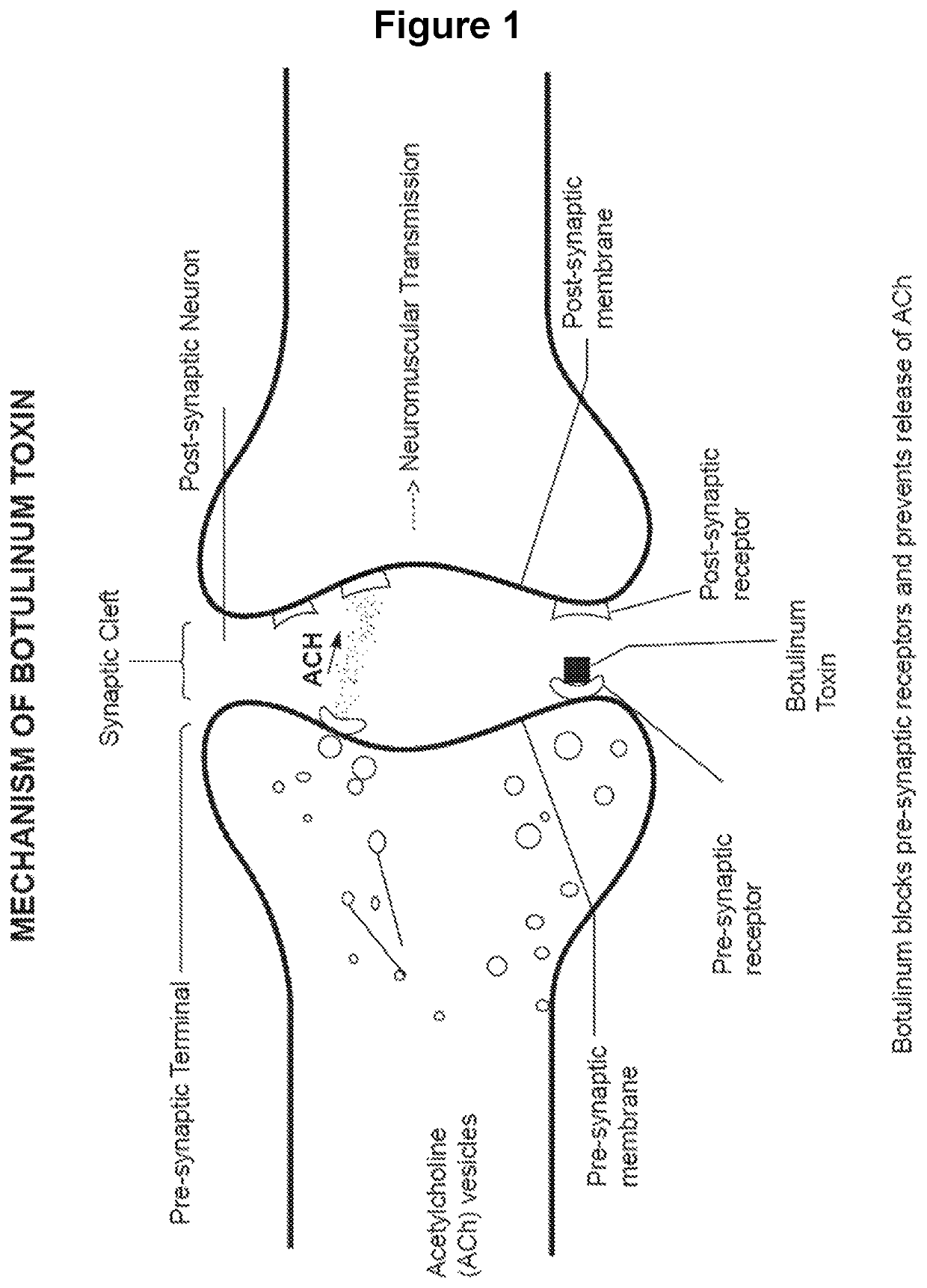
Treating of side-effects resulting from chemodenervation
a technology of chemodenervation and side effects, applied in the field of restoring neuromuscular transmission, can solve the problems of desensitization of the ach receptor, achieve the effects of preventing the ability of the ach to depolarize the receptor, prolonging the life of the ach, and increasing the concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
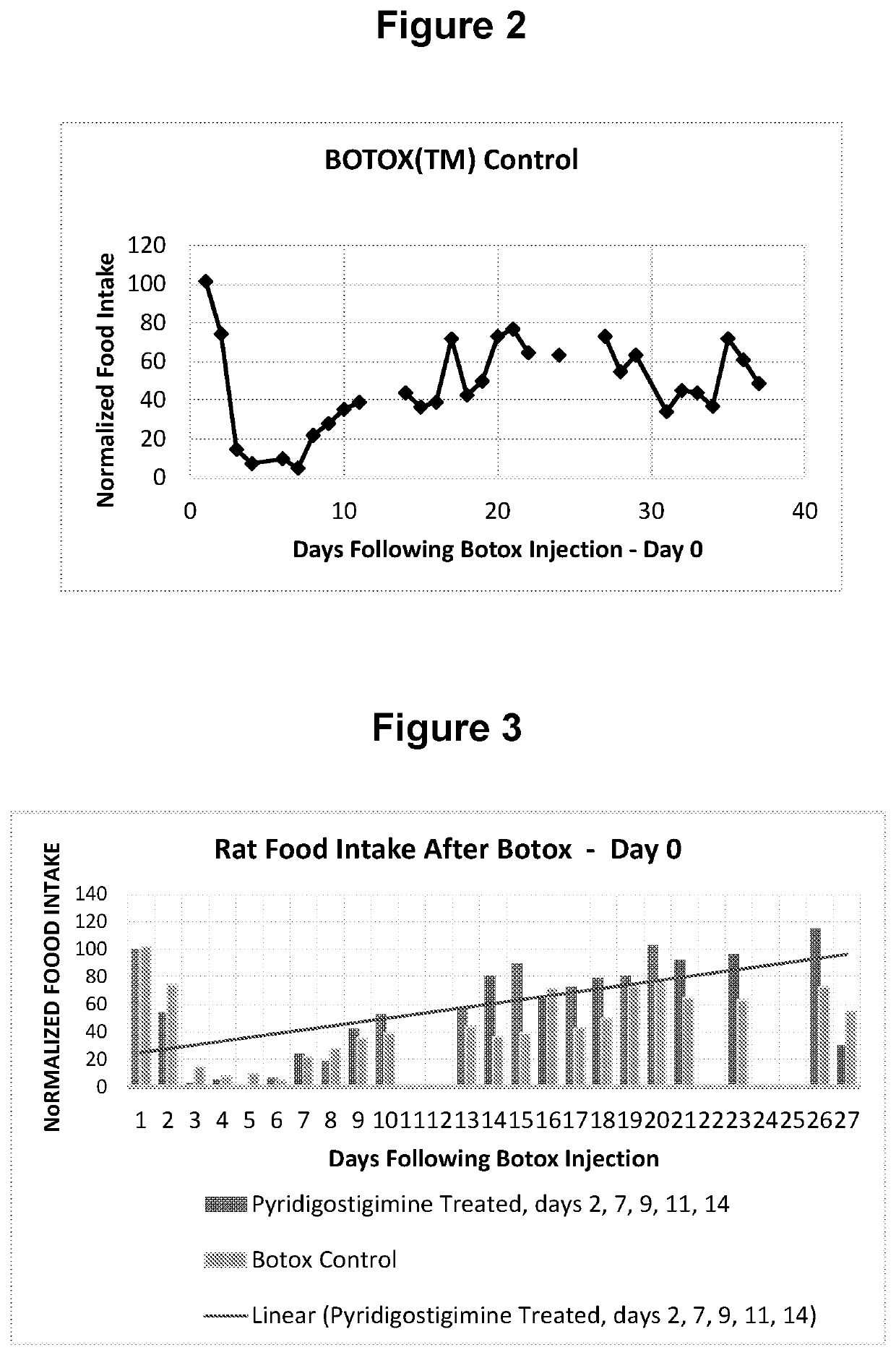
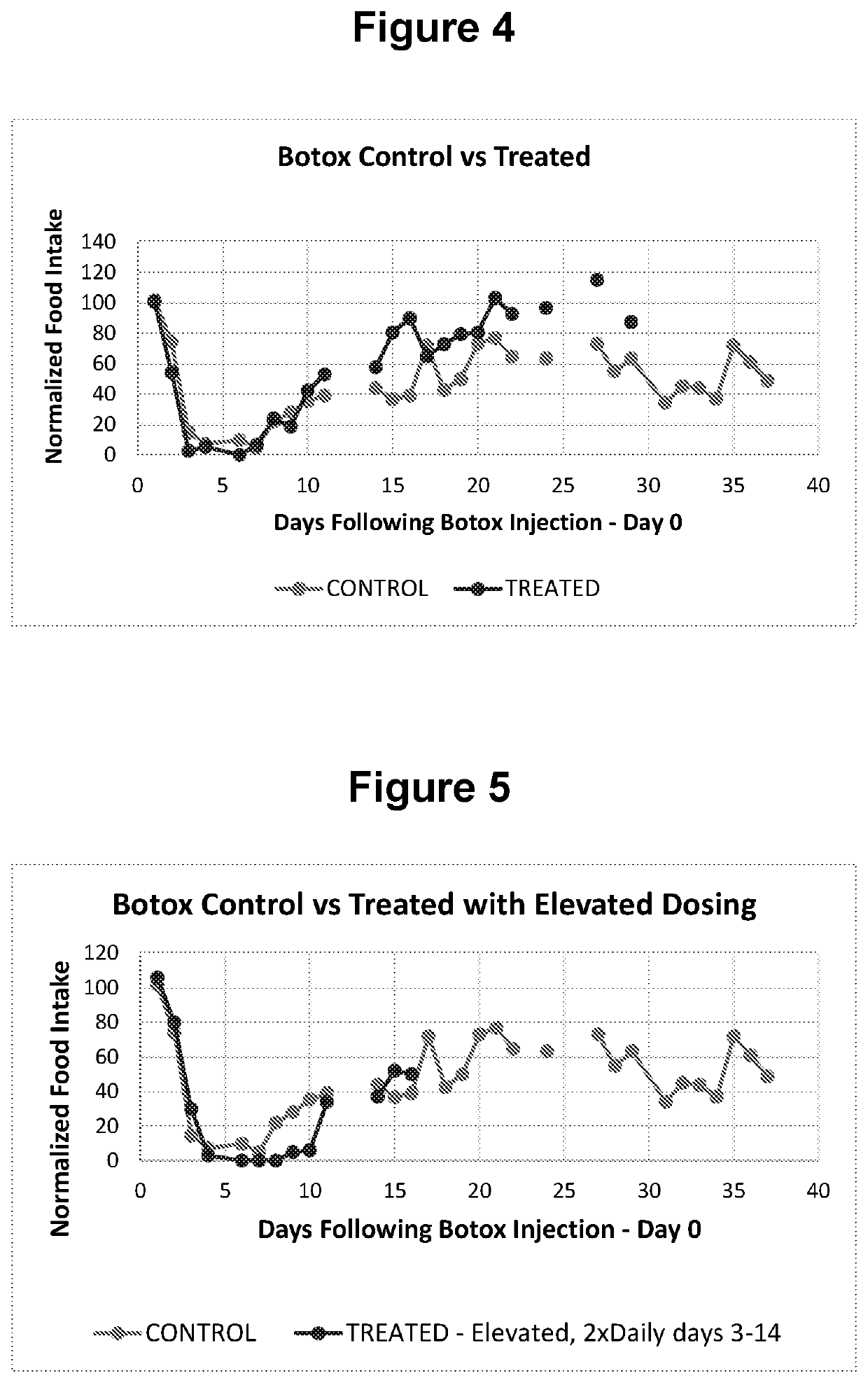
[0073]Animal Model and Methods
[0074]To demonstrate the efficacy of an anticholinesterase in accelerating the spontaneous recovery following botulinum toxin injections, the rat model outlined Moon et al. (Maxillofacial Plastic and Reconstructive Surgery (2015) 37:46) was adopted. In this case, botulinum toxin was injected into the masseter muscle of the rat and impending changes in food intake was monitored. The goal of the experiment was to cause paralysis in the jaw muscle of the rat using the botulinum neurotoxin. This was expected to negatively impact food intake and subsequently test the ability of comparable injections of anticholinesterase to accelerate the recovery process.
[0075]The test materials were products approved for injection. The botulinum toxin, BOTOX® (Allergan, Irvine, Calif., USA;), was reconstituted to the desired dose using saline for injection. The dose of BOTOX used in the experiments was 5 Units in 100 μl. For pryridostigimine, Regonol® (Sandoz Inc., Princet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| neuromuscular transmission | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com